An anti-glypican-3 antibody and an application thereof
A technology of phosphatidylinositol and proteoglycan, applied in the field of tumor immunotherapy or diagnosis, can solve the problems of curative effect, side effect, long-term survival, allergic reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
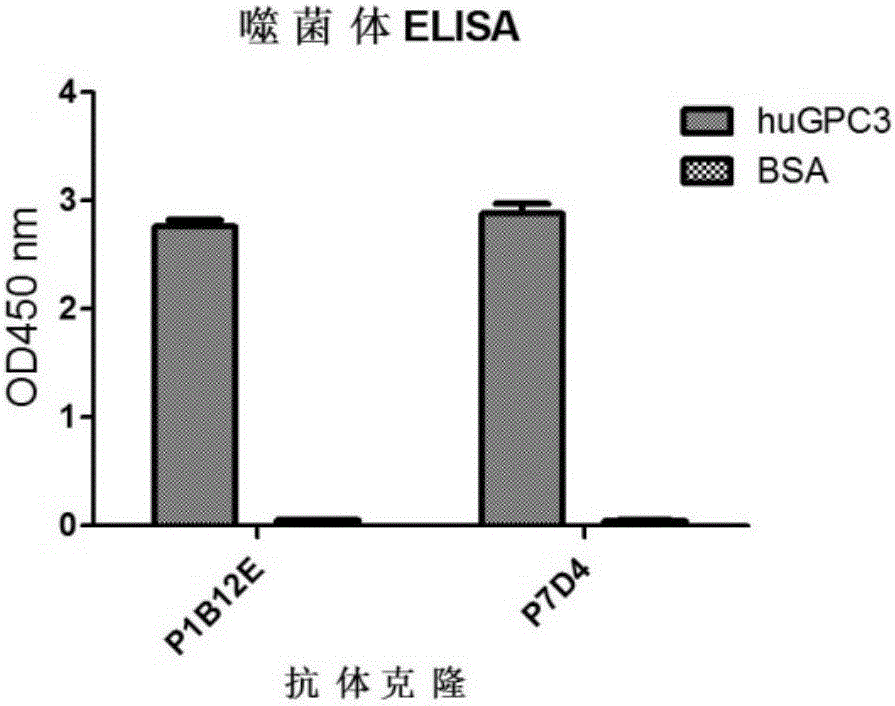
[0141] Example 1. Preparation of a specific single-chain antibody (scFv) that binds to human GPC3
[0142] 1.1 Screening of GPC3-specific binding antibodies based on phage display
[0143] Using phage display technology, human GPC3 (hereinafter referred to as huGPC3)-specific antibodies were screened from a fully human natural antibody library. For this purpose, 400ml of 2×YT / ampicillin medium was inoculated with glycerol bacteria (purchased from Shanghai Ruijin Biotechnology Co., Ltd.) displaying a natural library of fully human single-chain antibody by phage, so that the cell density reached OD 600 =0.1, shake culture at 37°C and 200rpm until the cell density reaches OD 600 = 0.5. use 10 12 pfu of M13KO7 helper phage (purchased from Invitrogen) was infected and incubated at 30° C. and 50 rpm for 30 minutes. After adding 50mg / L kanamycin, shake and culture at 37°C and 200rpm for 30 minutes, separate the precipitate by centrifugation (15 minutes, 1600×g, 4°C), resuspend in...
Embodiment 2
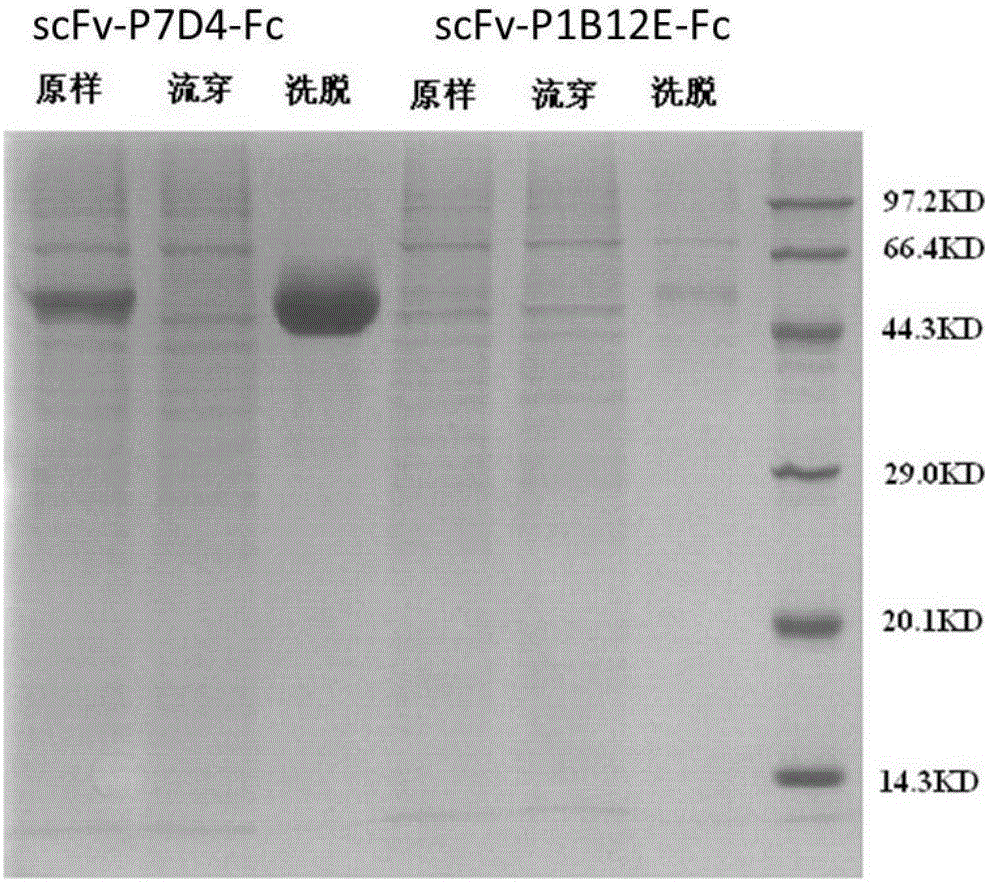
[0149] Example 2, Expression and purification of single-chain antibody against GPC3
[0150] The scFv was amplified from the plasmid (pCantab 5E-P1B12E) of the screened clone P1B12E using the primer pair V5-P1B12E-F (SEQ ID NO:5) and V5-P1B12E-R (SEQ ID NO:6) according to standard protocols - P1B12E fragment, scFv was amplified from the plasmid (pCantab 5E-P7D4) of clone P7D4 obtained by screening using the primer pair V5-P7D4-F (SEQ ID NO: 7) and V5-P7D4-R (SEQ ID NO: 8) -P7D4 fragment, through NheI / BamHI (purchased from NEB) double digestion, with T4DNA ligase (purchased from NEB) in the same with NheI / BamHI double digestion vector plasmid pCMV-V5-Fc (the vector is in the multiple cloning site Downstream fusion expresses the Fc fragment of human antibody IgG1, hereinafter referred to as V5-Fc, which was purchased from Shanghai Ruijin Biotechnology Co., Ltd.) and transformed into the host strain TOP10. The positive clones were picked and identified by PCR and confirmed by seq...
Embodiment 3
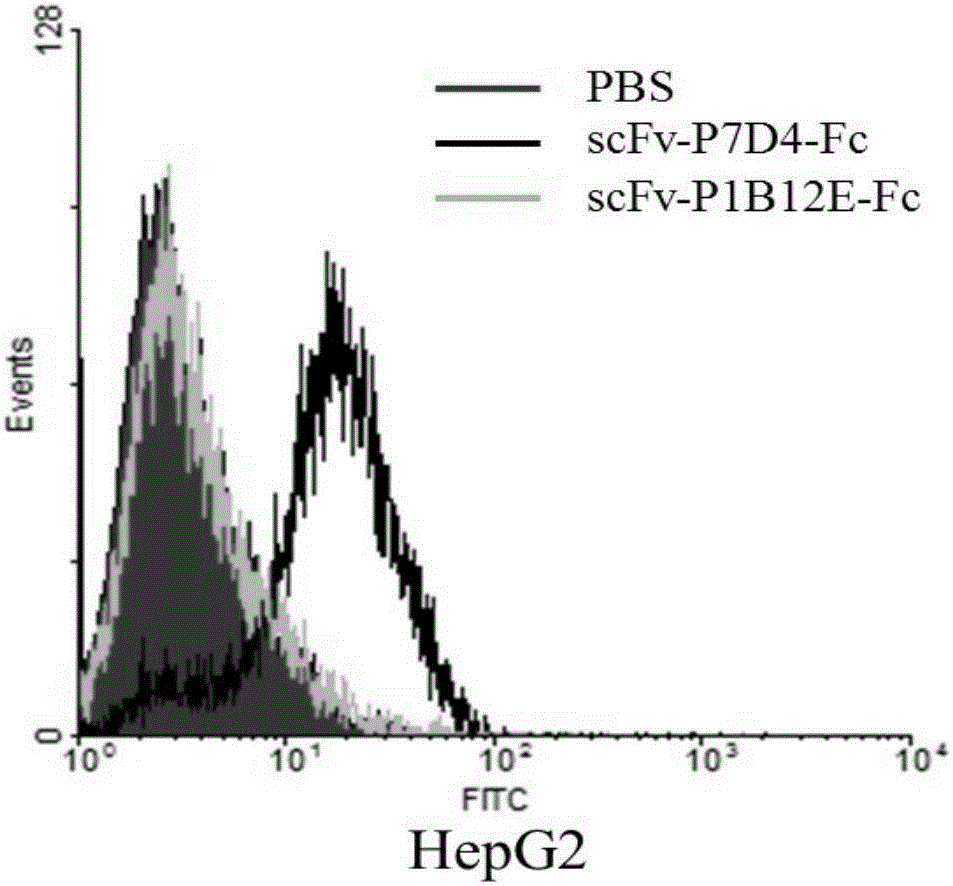
[0152] Example 3, flow cytometry analysis of the binding of each cell line to the anti-GPC3 single-chain antibody
[0153] The binding ability of the antibodies scFv-P1B12E-Fc and scFv-P7D4-Fc to the GPC3-positive liver cancer HepG2 cell line (ATCC) was analyzed by fluorescence activated cell sorting (FACS) (BD Company, FACSCalibur).
[0154] The specific method is as follows:
[0155] 1). Inoculate the HepG2 cell line of liver cancer in the logarithmic growth phase into a 6cm plate, the inoculated cell density is about 90%, and cultivate overnight in a 37°C incubator.
[0156] 2). Use 10mM EDTA to digest the cells, and collect the cells by centrifugation at 200g×5min. Take 1×10 6 ~1×10 7 The concentration per mL was resuspended in 1% phosphate buffered saline (NBS PBS) containing calf serum, and added to a flow-type tube in an amount of 100 ul / tube.
[0157] 3). Centrifuge at 200g×5min, discard the supernatant.
[0158] 4). The antibodies to be tested, scFv-P1B12E-Fc and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com