Synthesis and use of phenylpropionic acid derivatives
A derivative, phenylpropionic acid technology, applied in the prevention and treatment of diabetes, phenylpropionic acid derivatives as GPR120 receptor agonists, can solve problems such as obesity in humans and mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Preparation of intermediates
[0052] (4-fluoro-4'-methyl-[1,1'-biphenyl]-2-)methanol
[0053] At room temperature, 2-bromo-5-fluorobenzyl alcohol (410mg, 2mmol), p-tolueneboronic acid (299mg, 2.2mmol), anhydrous sodium carbonate (636mg, 6mmol) were dissolved in toluene (10mL), ethanol (5mL) , water (5mL) mixed solution. Under nitrogen protection, tetrakistriphenylphosphinepalladium (116mg, 0.1mmol) was added, and then raised to 70°C and stirred overnight. After the raw materials disappeared, the reaction liquid was cooled to room temperature, filtered with diatomaceous earth and washed with ethyl acetate. The filtrate was extracted with ethyl acetate and washed with saturated brine. The combined organic phases were dried over anhydrous sodium sulfate, desolvated and purified by column chromatography to obtain 267 mg of a yellow liquid with a yield of 62%. 1 H NMR (300MHz, CDCl 3 )δ7.33-7.14(m, 6H), 7.09-6.95(m, 1H), 4.57(s, 2H), 2.41(s, 3H), 1.99(s, 1H).
[0054] ...
Embodiment 1
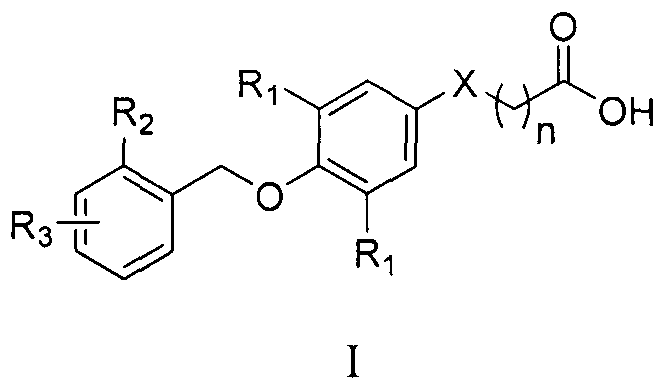
[0107] 3-(3,5-difluoro-4-((4-fluoro-4'-methyl-[1,1'-biphenyl]-2-)methoxy)phenyl)propanoic acid (I- 1)
[0108]
[0109] At room temperature, (4-fluoro-4'-methyl-[1,1'-biphenyl]-2-)methanol (160mg, 0.74mmol), 3-(3,5-difluoro-4-hydroxybenzene base) ethyl propionate (170 mg, 0.74 mmol), tributylphosphine (295 μL, 1.18 mmol) were dissolved in anhydrous toluene (10 mL). Under N2 protection, a solution of ADDP (298 mg, 1.18 mmol) in toluene (5 mL) was added dropwise to the above reaction solution. After the addition was complete, the reaction was stirred overnight at room temperature. After the raw materials disappeared, add an appropriate amount of n-hexane into the reaction flask, stir for 10 minutes, then filter with diatomaceous earth and wash the filter cake with n-hexane. After distilling off the solvent under reduced pressure, the product was purified by column chromatography to obtain a colorless liquid.
[0110] The colorless liquid was dissolved in methanol (2 mL), ...
Embodiment 2
[0112] 3-(4-((4-chloro-[1,1'-biphenyl]-2-)methoxy)-3,5-difluorophenyl)propanoic acid (I-2)
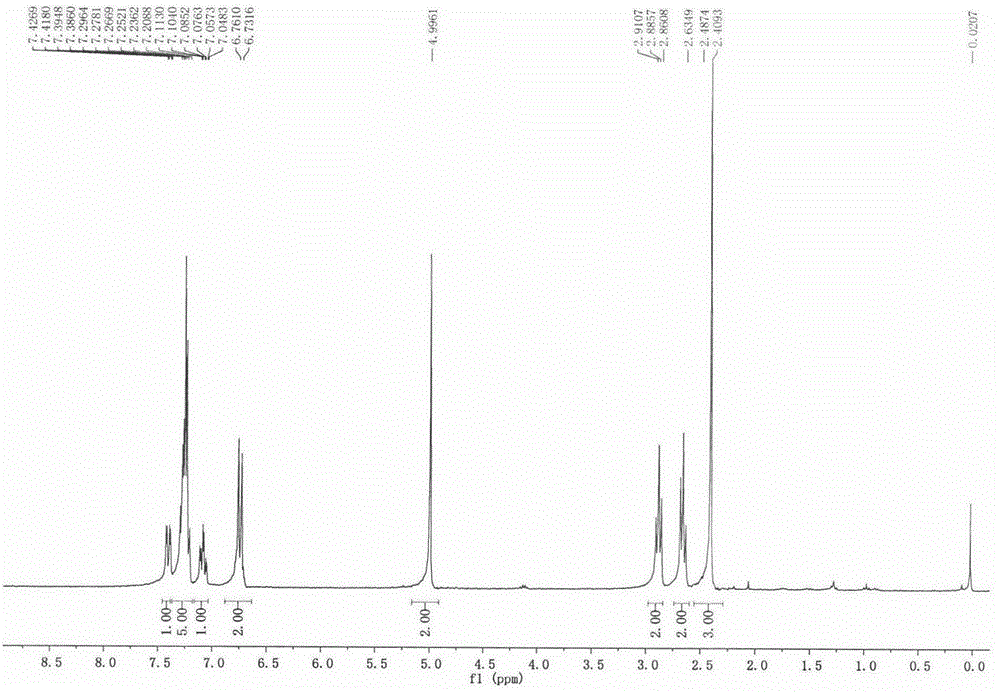
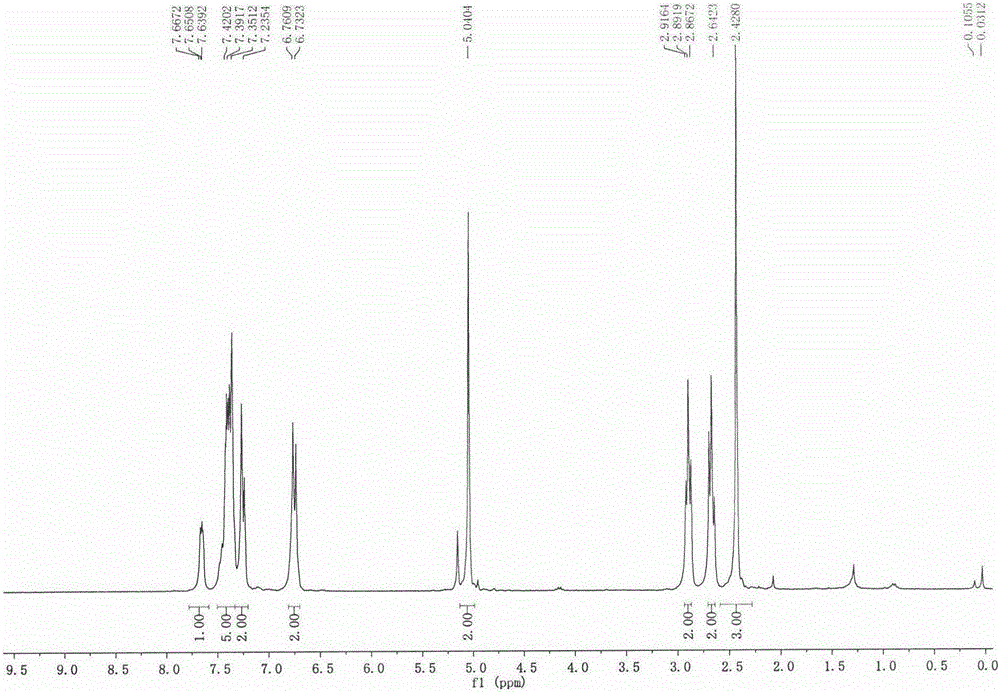
[0113] Prepare with reference to the method of Example 1. 1 H NMR (300MHz, CDCl 3 )δ7.68(d, J=1.0Hz, 1H), 7.44-7.35(m, 6H), 7.26(d, J=4.1Hz, 1H), 6.72(m, 2H), 4.97(s, 2H), 2.87(t, J=7.4Hz, 2H), 2.65(t, J=7.4Hz, 2H). 13C NMR (75MHz, CDCl 3 )δ178.56,157.62,157.54,155.19,154.32,154.24,140.40,139.11,136.39,136.28,136.17,135.55,135.18,133.38,131.26,130.18,130.01,129.86,129.62,129.33,129.15,128.39,128.26,128.08 , 127.56, 127.48, 127.20, 112.10, 112.00, 111.89, 111.80, 111.72, 73.22, 34.91, 29.70.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com