Pomalidomide impurity synthesis method
A technology of pomalidomide and a synthesis method, which is applied in the field of chemical pharmacy and achieves the effects of simple operation, improved accurate positioning and qualitative, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
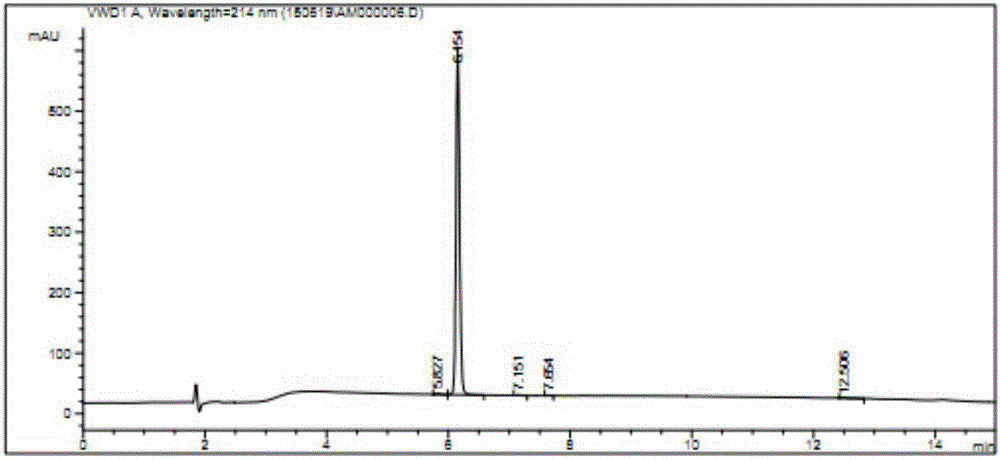
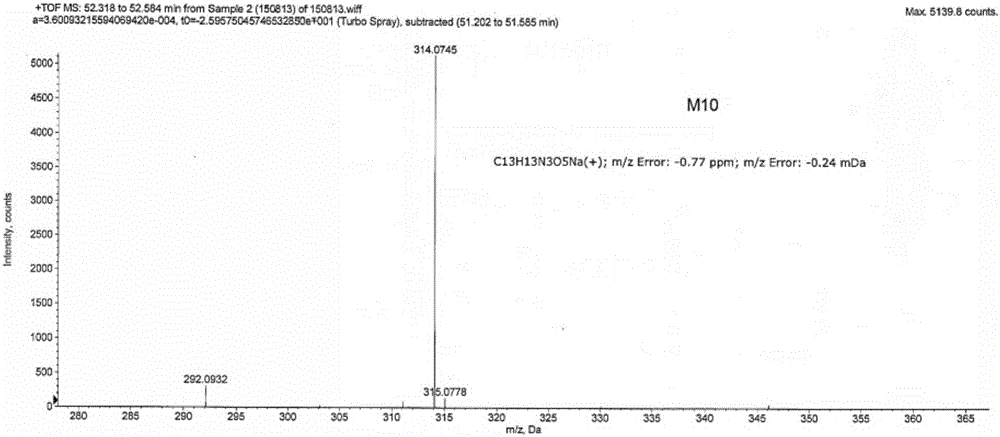
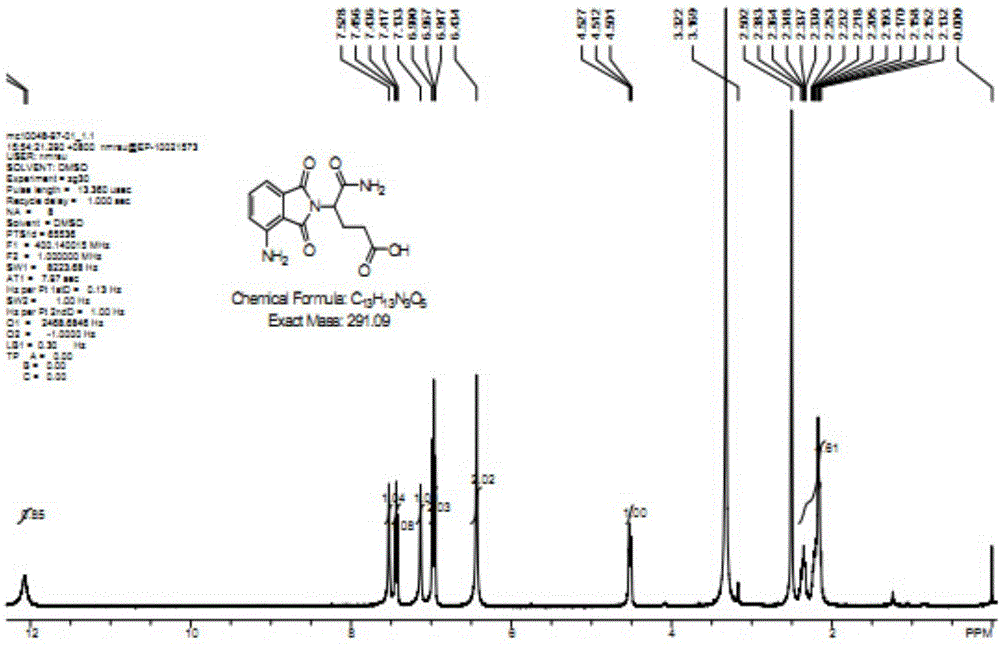
[0023] Example 1: Pomalidomide impurity M10: Synthesis of (S)-5-amino-4-(4-amino-1,3-dioxoisoindol-2-yl)-5-oxopentanoic acid
[0024] Combine 3-nitrophthalic anhydride (1) (25.0g, 129mmol), L-isoglutamine (18.5g, 126mmol), molecular sieve 92g) was mixed in N,N-dimethylformamide (400mL), and heated at 90°C for 16 hours. After the reaction is complete, the reaction solution is brought to room temperature, and the molecular sieve is filtered out. The filtrate was concentrated and concentrated to obtain a yellow solid, which was placed in a mixed solvent of methanol and water (V / V=3:26, 232ml) and stirred at room temperature overnight, and then filtered. The filtered solid was washed with water (100ml), and then dried in a vacuum oven (55°C) for 6 hours to obtain (S)-5-nitro-4-(4-amino-1,3-dioxoindole-2) -Yl)-5-oxopentanoic acid (2) (21.0 g, pale yellow solid, yield 53.0%). LC / MS=344.2[M+Na] + ,1H NMR(400MHz,DMSO-d 6 ): δ12.06(s,1H), 8.29(d,1H), 8.18(d,1H), 8.07(t,1H), 7.62(s,1H),...
Embodiment 2
[0029] Example 2: Pomalidomide impurity M10: Synthesis of (S)-5-amino-4-(4-amino-1,3-dioxoisoindol-2-yl)-5-oxopentanoic acid
[0030] Combine 3-nitrophthalic anhydride (1) (25.0g, 129mmol), L-isoglutamine (17.5g, 119mmol), molecular sieve ( 50g) was mixed in N,N-dimethylformamide (266 mL), and heated at 80°C for 15 hours. After the reaction is complete, the reaction solution is brought to room temperature, and the molecular sieve is filtered out. The filtrate was concentrated and concentrated to obtain a yellow solid, which was placed in a mixed solvent of methanol and water (V / V=3:26, 232ml) and stirred at room temperature overnight, and then filtered. The filtered solid was washed with water (100ml), and then dried in a vacuum oven (50°C) for 4 hours to obtain (S)-5-nitro-4-(4-amino-1,3-dioxoindole-2) -Yl)-5-oxopentanoic acid (2) (20.0 g, pale yellow solid, yield 50.5%).
[0031] The above (S)-5-nitro-4-(4-amino-1,3-dioxoisoindol-2-yl)-5-oxopentanoic acid (2) (21.0g, 65.5mmol)...
Embodiment 3
[0032] Example 3: Pomalidomide Impurity M10: Synthesis of (S)-5-amino-4-(4-amino-1,3-dioxoisoindol-2-yl)-5-oxopentanoic acid
[0033] Combine 3-nitrophthalic anhydride (1) (25.0g, 129mmol), L-isoglutamine (37.5g, 255mmol), molecular sieve ( 125g) was mixed in N,N-dimethylformamide (798 mL), and heated at 120°C for 20 hours. After the reaction is complete, the reaction solution is brought to room temperature, and the molecular sieve is filtered out. The filtrate was concentrated and concentrated to obtain a yellow solid, which was placed in a mixed solvent of methanol and water (V / V=3:26, 232ml) and stirred at room temperature overnight, and then filtered. The filtered solid was washed with water (100ml) and then dried in a vacuum oven (60°C) for 8 hours to obtain (S)-5-nitro-4-(4-amino-1,3-dioxoindole-2) -Yl)-5-oxopentanoic acid (2) (20.5 g, pale yellow solid, yield 51.8%).
[0034] The above (S)-5-nitro-4-(4-amino-1,3-dioxoisoindol-2-yl)-5-oxopentanoic acid (2) (21.0g, 65.5mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com