A kind of perfluoropolyether alkoxysilane compound and its synthesis method
A technology of perfluoropolyether alkoxysilane, synthesis method, applied in polyurea/polyurethane coatings, paints containing biocide, coatings, etc., can solve the problem of inability to provide adhesion, wear resistance and durability, The problem of small number ratio and poor antifouling performance can achieve the effect of excellent antifouling performance, solving insufficient wear resistance, excellent anti-fingerprint antifouling performance and wear resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
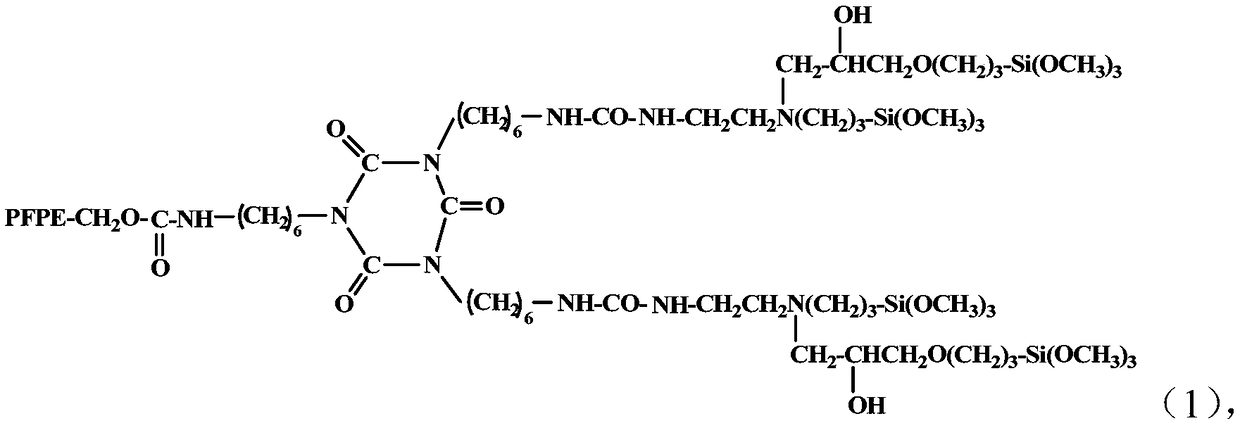
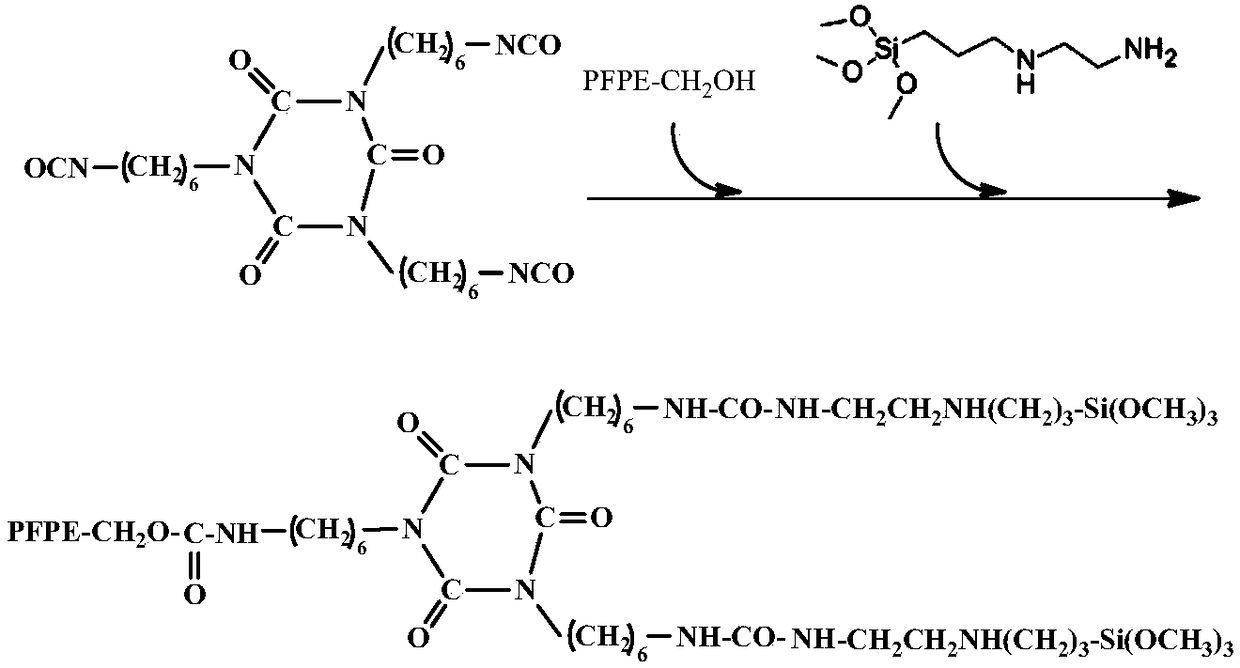
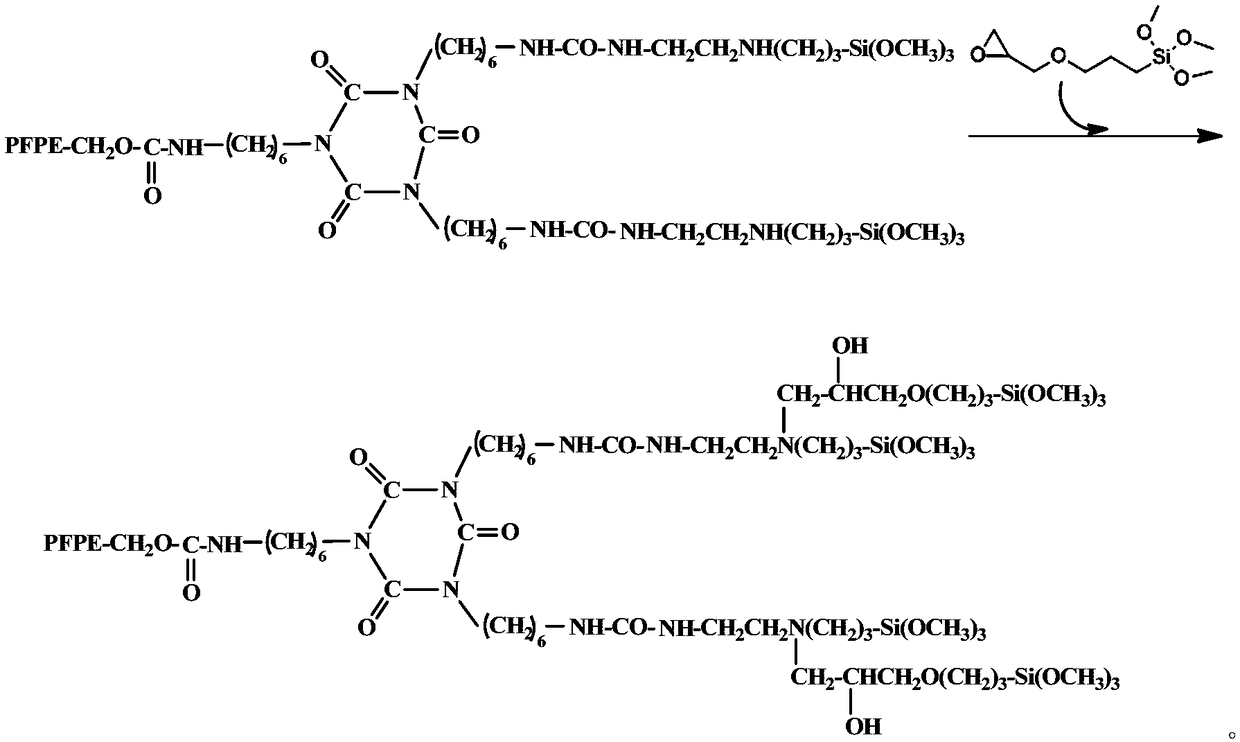
[0027] A kind of synthetic method of perfluoropolyether alkoxysilane compound, comprises the steps:
[0028] (a) Dilute the HDI trimer with a solvent and add it to the reaction vessel, and then dilute the mixed homogeneous mixture of hydroxyl-terminated perfluoropolyether and 3-(2-aminoethyl)aminopropyltrimethoxysilane with a solvent under stirring Gradually add it dropwise, and after the dropwise addition, stir and keep warm at the reaction temperature for 6 hours.
[0029] (b) Add dropwise 3-(2,3-epoxypropoxy)propyltrimethoxysilane (KH560) with the same molar number as 3-(2-aminoethyl)aminopropyltrimethoxysilane, After continuing to react at the reaction temperature for 6 hours, the perfluoropolyether alkoxysilane compound is obtained.
[0030] The reaction molar ratio of hydroxyl-terminated perfluoropolyether, 3-(2-aminoethyl)aminopropyltrimethoxysilane, KH560, and HDI trimer is 1:2:2:1, and the molecular weight of hydroxyl-terminated perfluoropolyether is MW For 2500-500...
Synthetic example 1
[0036] In a 150ml three-necked flask equipped with a constant temperature magnetic stirrer, a constant pressure dropping funnel and a thermometer, add 25ml of fluoroether solvent and 2.52g (5mmol) of HDI trimer, stir, dilute and dissolve, and then mix the terminal hydroxyl group homogeneously with fluoroether solvent. 12.5g (5mmol, molecular weight MW=2500) of perfluoropolyether and 2.22g (10mmol) of 3-(2-aminoethyl)aminopropyltrimethoxysilane were gradually added dropwise. After the dropwise addition, stirred and kept at 30°C for reaction After 6 hours, add KH560 2.36g (10mmol) dropwise, and after the dropwise addition, stir and keep warm at 30°C for 6 hours to remove the solvent under reduced pressure to obtain the product. The structural formula of the obtained product is:
[0037]
[0038] Wherein PFPE molecular weight MW=2500.
Synthetic example 2
[0040] In a 150ml three-necked flask equipped with a constant temperature magnetic stirrer, a constant pressure dropping funnel and a thermometer, add 25ml of fluoroether solvent and 2.52g (5mmol) of HDI trimer, stir, dilute and dissolve, and then mix the terminal hydroxyl group homogeneously with fluoroether solvent. The mixture of 15g (5mmol, molecular weight MW=3000) of perfluoropolyether and 2.22g (10mmol) of 3-(2-aminoethyl)aminopropyltrimethoxysilane was gradually added dropwise, and the mixture was stirred and kept at 30°C after the dropwise addition for 6 Hours, then add 2.36g (10mmol) of KH560 dropwise, and after the dropwise addition, stir and keep warm at 30°C for 6 hours to remove the solvent under reduced pressure to obtain the product. The structural formula of the resulting product is:
[0041]
[0042] Wherein PFPE molecular weight MW=3000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com