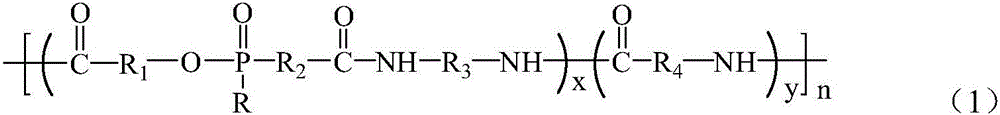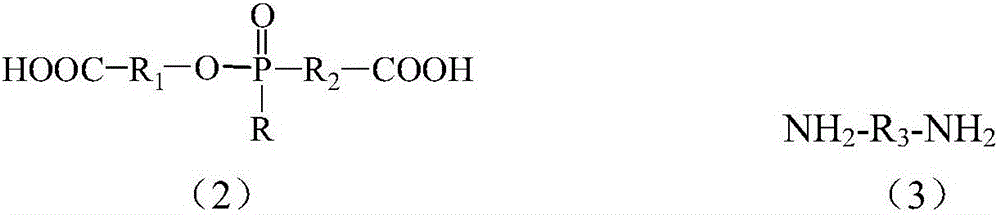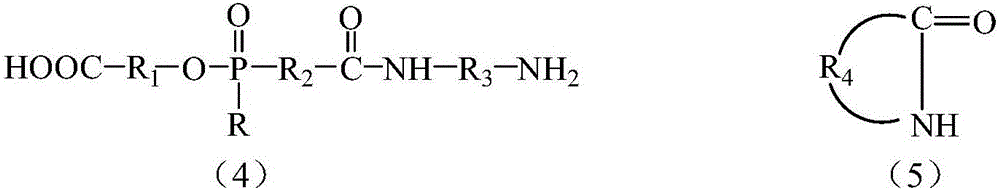Organophosphorus flame-retarded copolymeric nylon composition and preparation method thereof
A technology for copolymerizing nylon and organophosphorus, applied in the field of organophosphorus flame-retardant copolymerized nylon composition and its preparation, flame-retardant copolymerized nylon composition and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The preparation method of flame-retardant nylon composition of the present invention comprises:
[0066] (A) reacting organophosphorus flame retardant monomers or their derivatives with diamine monomers to make excess amino groups and prepare salt solutions;
[0067](B) allowing the reaction product of step (A) to react with a lactam in the presence of an inorganic nanomaterial to generate a flame retardant nylon composition; or
[0068] (B') Generate organophosphorus flame-retardant copolymer nylon without the presence of inorganic nanomaterials, and then blend organophosphorus flame-retardant copolymer nylon and inorganic nanomaterials.
[0069] In step (A), preferably, the flame retardant reacts with the diamine monomer in a molar ratio of 1:0.1-20, preferably 1:0.5-10, more preferably 1:1-2.
[0070] In step (A), preferably, the diamine monomer is slightly excessive, so that the pH of the prepared salt solution is 7-9. The reaction temperature rose from room tempe...
Embodiment 1
[0089] Stir and react the flame retardant monomer and hexamethylenediamine in a small amount of water at a molar ratio of 1:1.3 at 50°C for 0.5h, adjust the pH of the solution to 7.5, and dry to form a salt. Caprolactam monomer, salt obtained above (accounting for 6% of the total mass of caprolactam monomer, the same below), nano-montmorillonite accounting for 4wt% of caprolactam monomer, catalyst sodium hypophosphite each accounting for 1wt‰, antioxidant Agent p-phenylenediamine, molecular weight regulator benzoic acid, and 3% water were added to the reactor, vacuumized, nitrogen filled three times, and finally the internal pressure of the reactor was kept at 0.25MPa. Heat the reactor and keep stirring at a high speed. When the temperature of the reactor reaches 215° C. and the pressure is 1.75 MPa, keep the temperature and pressure constant for 2 hours. Then the temperature was raised to 260°C, and the pressure was released to normal pressure within 1.5 hours. Vacuumize to ...
Embodiment 2
[0092] Stir and react the flame retardant monomer and hexamethylenediamine in a small amount of water at a molar ratio of 1:1.1 at 55°C for 40 minutes, adjust the pH of the solution to 7.3, and dry to form a salt. The decanolactam monomer, the salt obtained above (accounting for 7% of the total mass of the decanolactam monomer), the nano talc accounting for 4wt%, the catalyst toluenesulfonic acid each accounting for 1‰, and the antioxidant 2 , 6-di-tert-butyl-4-methylphenol and molecular weight regulator adipic acid were added to the reactor, vacuumed and filled with nitrogen three times, and finally the pressure inside the reactor was kept at 0.2 MPa. Heat the reactor and keep stirring at a high speed. When the temperature of the reactor reaches 220° C. and the pressure is 1.9 MPa, keep the temperature and pressure constant for 2 hours. Then the temperature was raised to 270°C, and the pressure was released to normal pressure within 2 hours. Vacuumize to -0.08MPa. After the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Flammability rating | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com