Improved method for synthesizing clethodim
A synthetic method, the technology of clethodim, applied in the field of improved clethodim synthesis, can solve problems such as non-compliance, unfavorable industrial production, and affecting the quality of clethodim, and achieve the effects of good quality, easy industrial production, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Put 43 grams (0.25mol) of compound (Ⅲ) and 100 grams of toluene into a 500ml three-necked flask, then add 0.43 grams of methyl propionate and 33 grams (0.25mol) of dimethyl malonate, cool to room temperature, drop 65 grams (0.3 mol) of sodium methoxide methanol solution with a content of 25%, and then the mixture was stirred overnight at room temperature. After the completion of the reaction was determined by thin layer chromatography, heating and distillation was added and 100 grams of toluene was added until there was no alcohol to obtain the compound ( IV). After cooling, 23 g (0.25 mol) of propionyl chloride was added dropwise at 60-70° C. After the drop, the temperature was raised to 90° C. and kept for 2 hours to obtain compound (Ⅴ). Adjust the pH to 7 with triethylamine, then add 2 g of 4-dimethylaminopyridine, and react at 90° C. for 4 hours to obtain compound (VI). Then add 100 g of water and 80 g (0.6 mol) of 30% liquid caustic soda, react at 90°C for 4 hours...
Embodiment 2
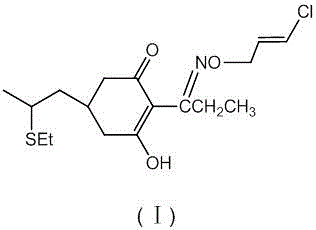
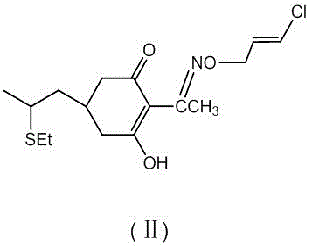
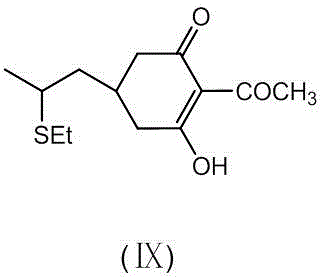
[0028] Put 43 g (0.25 mol) of compound (Ⅲ), 100 g toluene and 33 g (0.25 mol) of dimethyl malonate into a 500 ml three-necked flask, cool to room temperature, and add dropwise 25% sodium methoxide methanol solution 65 g (0.3 mol), then 1.5 g of methyl propionate was added, and the mixture was stirred overnight at room temperature, and the reaction was complete as determined by thin layer chromatography. Subsequent steps were continued as described in Example 1. 52.7 g of compound (Ⅷ) was obtained in the middle, and by high-performance liquid chromatography analysis, the purity was 91.0%, the yield was 71%, and the ratio of compound (IX) was 0.25% by weight. Finally, 69.4 g of compound (I) was obtained. Through high-performance liquid chromatography analysis, the purity was 92.8%, the total yield was 71.6%, and the proportion of compound (II) was 0.25% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com