Pentazole composite salt and preparation method thereof
A compound salt and phenyl pentazole technology, applied in the direction of organic chemistry, to achieve the effect of huge application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
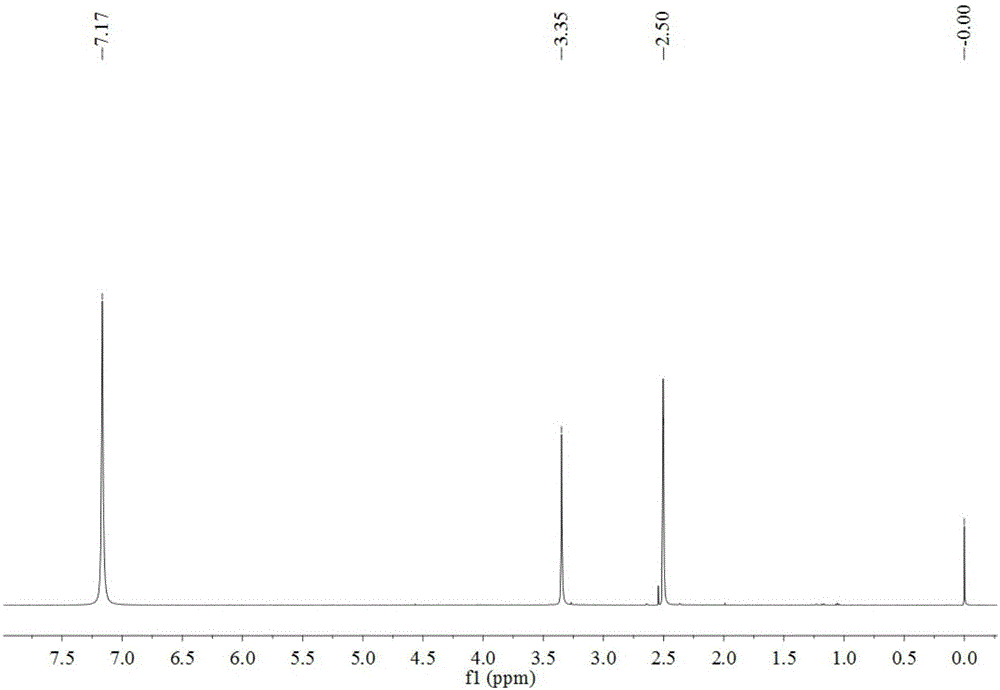
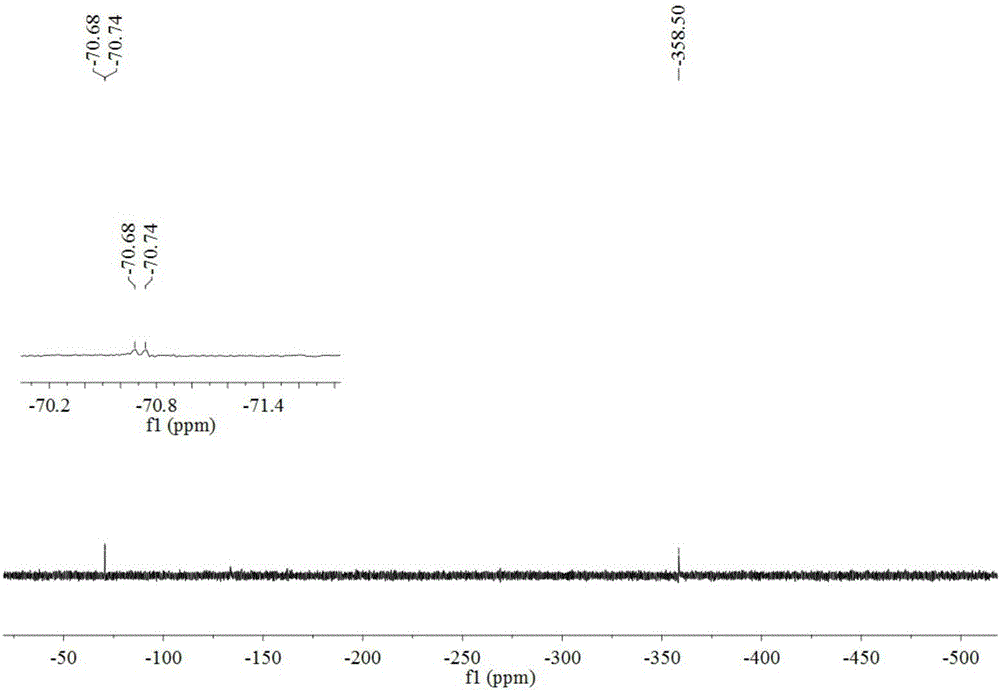
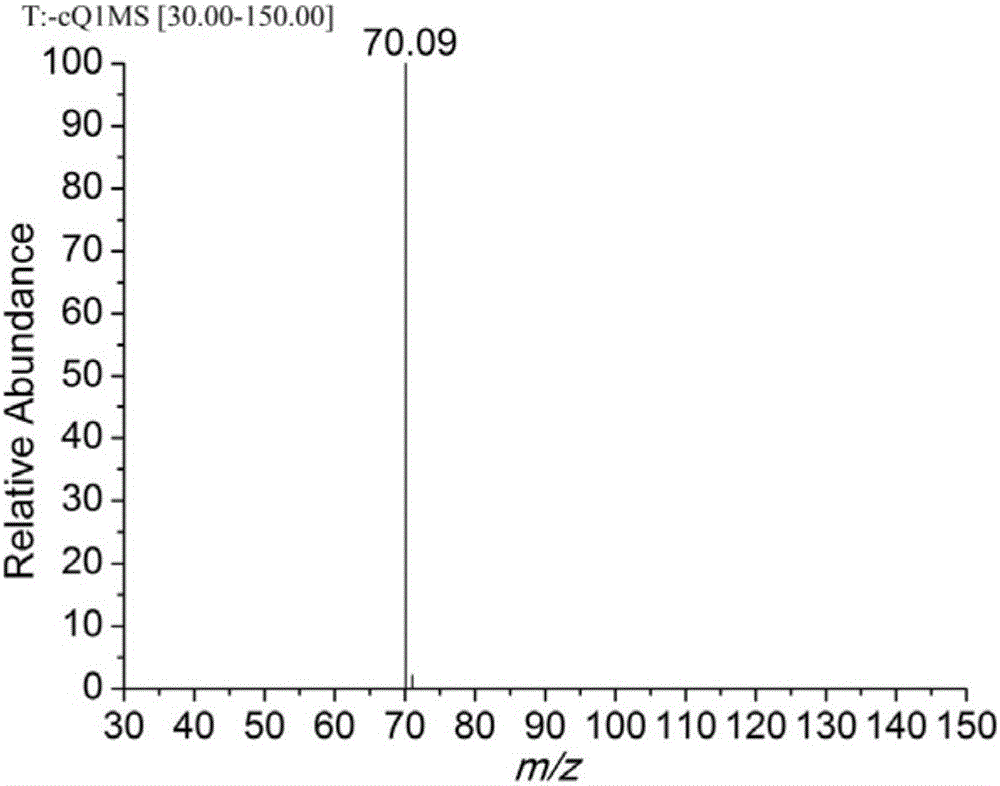
[0031] In a single-necked flask, 4-hydroxyphenylpentazole (3mmol) was dissolved in a mixed solvent of methanol and acetonitrile (v / v, 1 / 1), and under the reaction condition of -45°C, ferrous glycinate (6mmol) was added Aqueous methanol (v / v, 1 / 4) (pre-cooled to -45°C), stirred for 30min, then added m-chloroperoxybenzoic acid (12mmol) in methanol (precooled to -45°C), reacted for 36h. Filtrate, remove most of the solvent by rotary evaporation, dissolve the obtained product in ethyl acetate, add water for liquid extraction, combine the water phases, and rotary evaporate through the column to obtain 21.2 mg of pentazole compound salt, with a yield of 12.08%.
Embodiment 2
[0033]In a one-necked flask, dissolve 4-hydroxyphenylpentazole (3mmol) in a mixed solvent of methanol and acetonitrile (v / v, 1 / 1), and add ferrous sulfate heptahydrate (6mmol) under the reaction condition of -45°C ) in methanol aqueous solution (v / v, 1 / 5) (pre-cooled to -45°C), stirred for 30min, then added m-chloroperoxybenzoic acid (12mmol) in methanol solution (pre-cooled to -45°C), and reacted for 36h . Filtrate, remove most of the solvent by rotary evaporation, dissolve the obtained product in ether, add water for liquid extraction, combine the water phases, and rotary evaporate through the column to obtain 14.5 mg of pentazole compound salt, with a yield of 8.26%.
Embodiment 3
[0035] In a single-necked flask, 4-hydroxyphenylpentazole (3mmol) was dissolved in a mixed solvent of methanol and acetonitrile (v / v, 1 / 1), and under the reaction condition of -45°C, ferrous chloride tetrahydrate ( 6mmol) of methanol aqueous solution (v / v, 1 / 6) (pre-cooled to -45°C), stirred for 30min, then added m-chloroperoxybenzoic acid (12mmol) in methanol solution (pre-cooled to -45°C), and reacted 36h. Filtrate, remove most of the solvent by rotary evaporation, dissolve the obtained product in ethyl acetate, add water for liquid extraction, combine the aqueous phases, and rotary evaporate through the column to obtain 12.5 mg of pentazole compound salt with a yield of 7.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com