A kind of recombinant bacteria producing alkaline pectinase and its application
A pectinase and alkaline technology, applied in the field of genetic engineering, can solve the problems of low expression level, the yield of alkaline pectinase cannot be further improved, and the industrial production of alkaline pectinase is restricted, and the effect of high expression is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Construction of embodiment 1 recombinant bacteria Pichia pastoris GS115 / N185Q
[0042] Using the gene K314Mopt (application number 201610170070.0, published in the patent application on July 13, 2016) as the starting control gene, the alkaline pectinase gene PGL / N185Q, then design primers, obtain the alkaline pectinase gene N185Q (sequence shown in SEQ ID NO.1) by PCR method, clone it into the expression vector pPIC9K, obtain the recombinant plasmid pPIC9K-N185Q, transform the recombinant vector Pichia pastoris GS115, the recombinant strain Pichia pastoris GS115-pPIC9K-N185Q was obtained after screening and identification.
[0043] Primers are as follows:
[0044] PGL-F: GCTGAAGCTTACGTAGAATTCGCTGATTTGGGTCATCAAACACTTG
[0045] PGL-R: AAGGCGAATTAATTCGCGGCCGCTTAGTTCAATTTTCCAGCACCTGCT
[0046] The gene was transferred into Pichia pastoris cells by electroporation. The specific steps are as follows: Pick a single colony of yeast recipient bacteria and inoculate it in 25...
Embodiment 2
[0047] Example 2 Construction of Pichia pastoris GS115 / N185Q-ERO1-UBC1
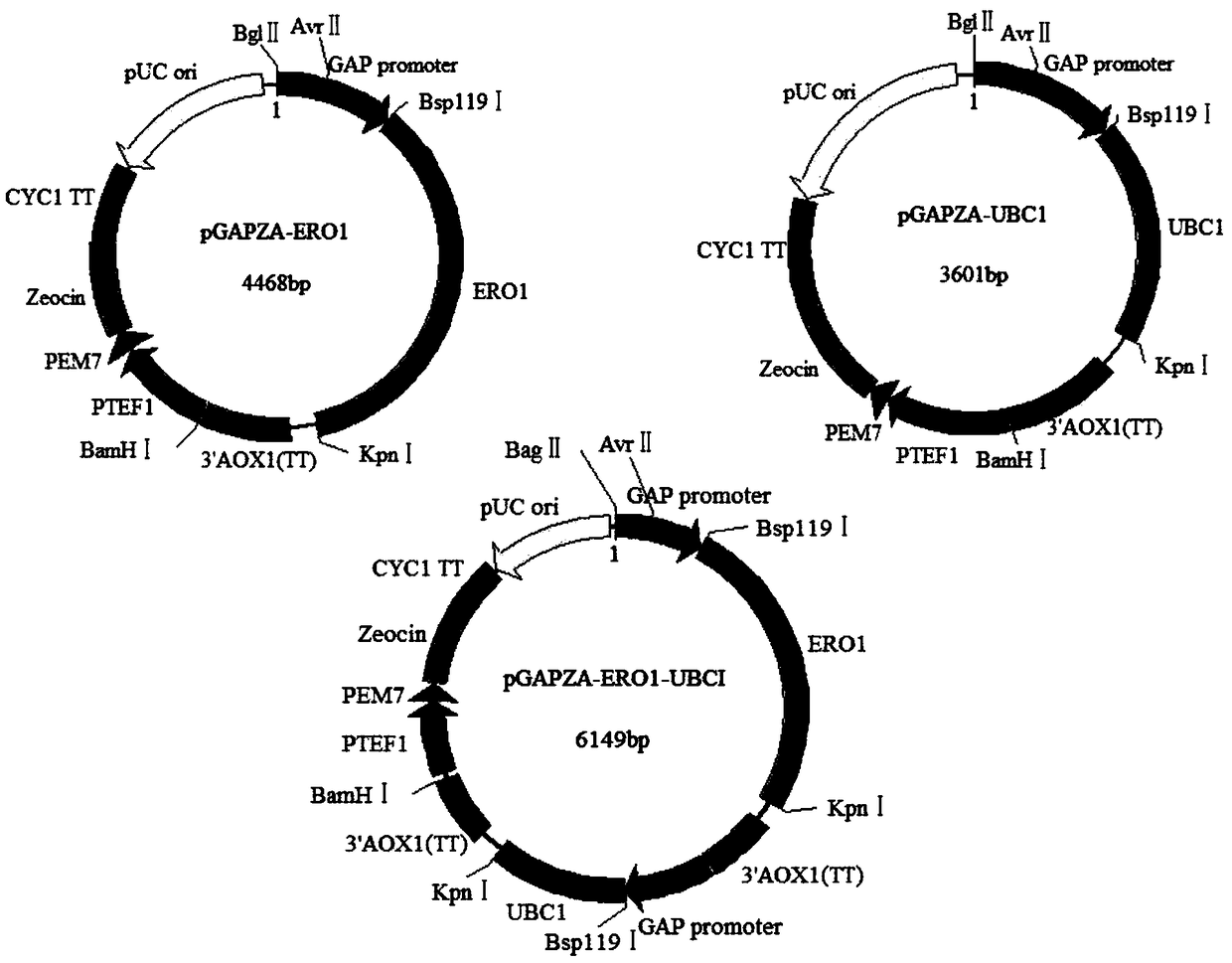
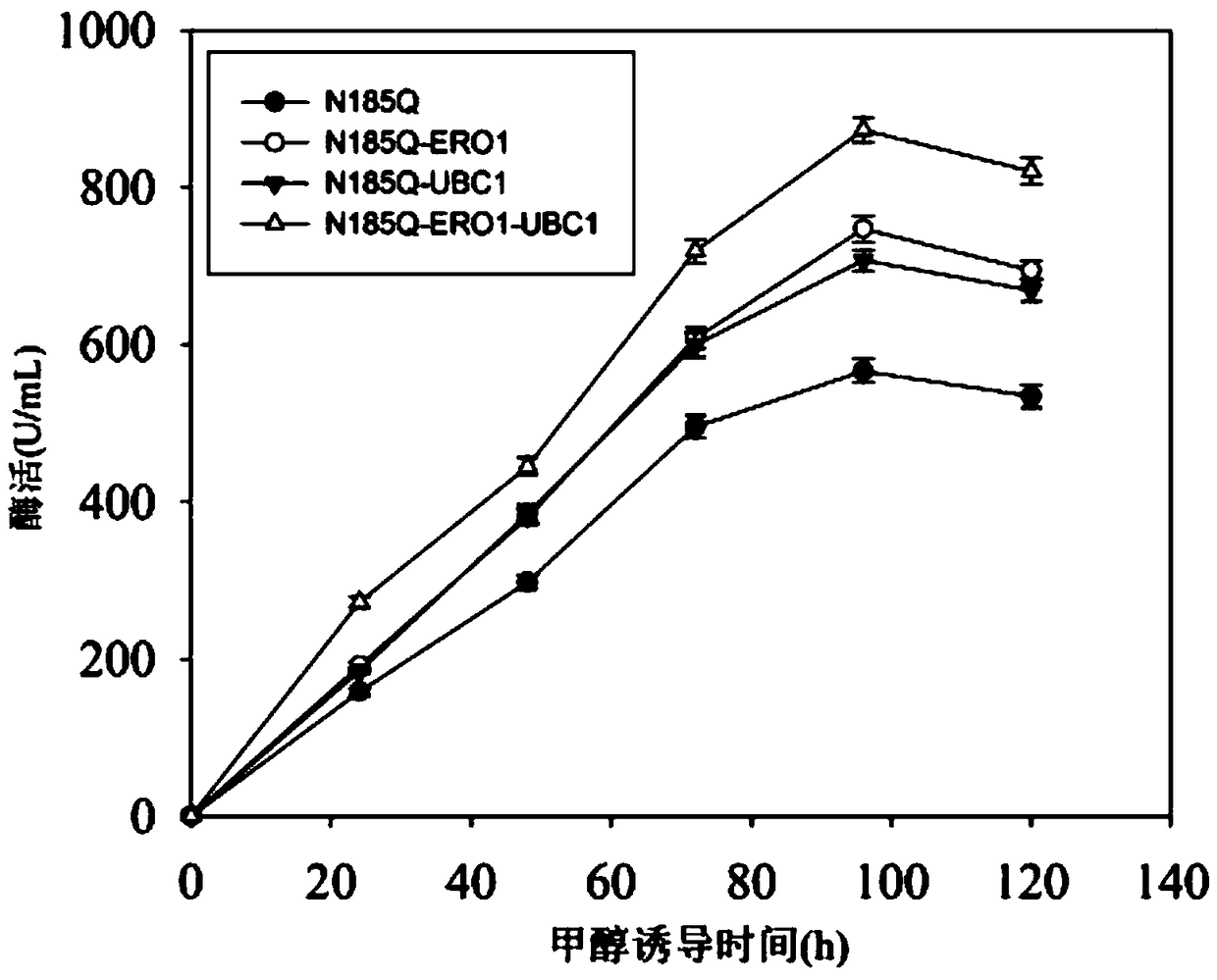
[0048] Extract Pichia pastoris RNA, reverse transcribe it into cDNA, use cDNA as a template, design primers, obtain ERO1 and UBC1 genes by PCR, clone them into the expression vector pGAPZA, and obtain recombinant plasmids pGAPZA-ERO1 and pGAPZA-UBC1, Then construct the co-expression vector pGAPZA-ERO1-UBC1 based on the isotail enzyme effect of BglⅡ and BamHI (the schematic diagram of cloning expression plasmid is shown in the appendix figure 1 ), the recombinant vector pGAPZA-ERO1-UBC1 was transformed into Pichia pastorisGS115-pPIC9K-N185Q (prepared in Example 1), and the co-expression recombinant strain Pichia pastorisGS115 / N185Q-ERO1-UBC1 was obtained through screening and identification.
[0049] Primers are as follows:
[0050]
[0051] The transformation of Pichia pastoris was performed by electroporation.
[0052] The specific steps are as follows: Pick a single colony of yeast recipient bacter...
Embodiment 3
[0053] Embodiment 3 co-expression genetic engineering strain shake flask fermentation culture
[0054] Cultivation method: After seed activation, the strain was inoculated into the basic fermentation medium YPD, cultured at 30°C and 220rpm for 14h, then transferred to the optimized growth medium BMGY and cultured at 30°C and 220rpm for 24h, and then the strain Transfer to the induction medium BMMY at 22-28°C, 220rpm and add methanol at a final concentration of 10-20mL / L every 24h to induce the expression of alkaline pectinase.
[0055] Select Beyontian SDS-PAGE gel electrophoresis kit to prepare 12% separating gel and 5% stacking gel. For specific operation methods, please refer to the product manual. The sample was mixed with 5× loading buffer at a volume ratio of 4:1, boiled in water bath for 10 min, and loaded after cooling. During electrophoresis, the constant voltage is 80V. After the indicator enters the separation gel, the voltage is adjusted to 150V, and the electroph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com