Preparation of mesothelin chimeric antigen receptor modified T cells and application of T cells in pancreatic cancer treatment
A chimeric antigen receptor, pancreatic cancer technology, applied to genetically modified cells, cells modified by introducing foreign genetic material, receptors/cell surface antigens/cell surface determinants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
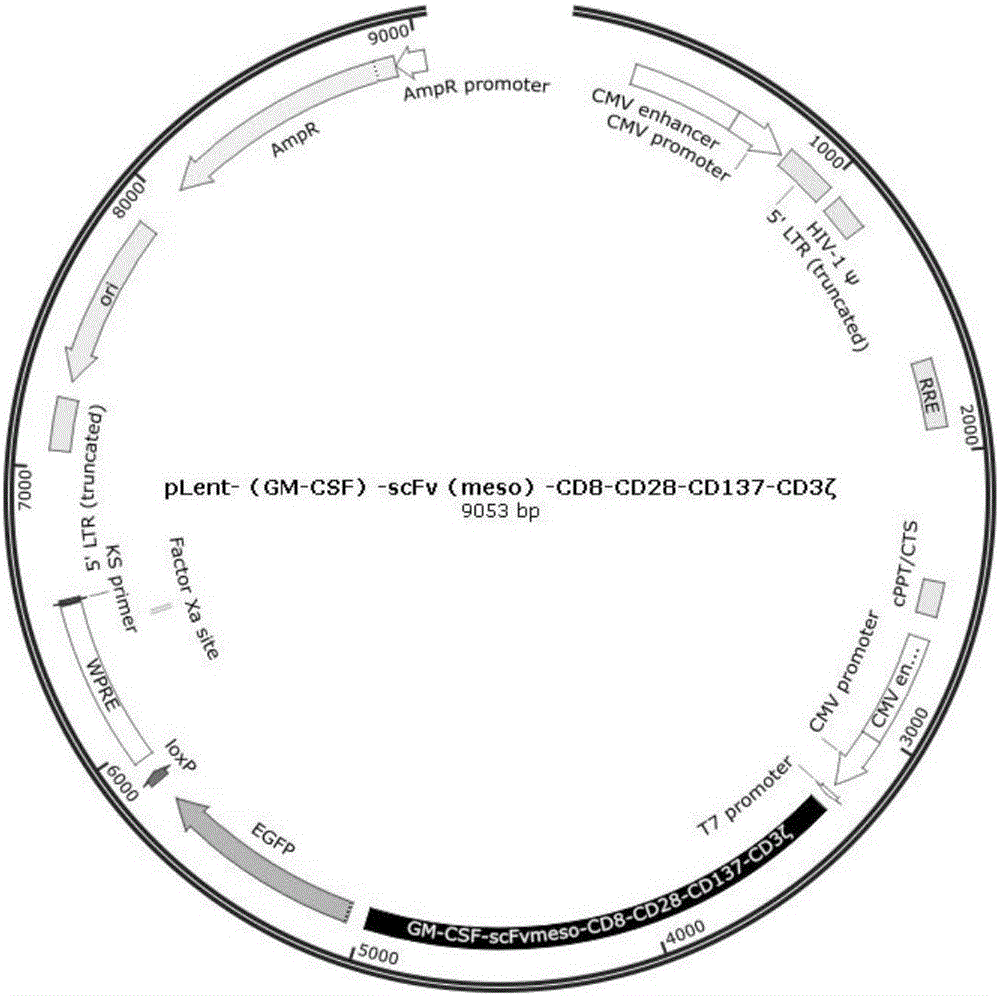
[0049] Example 1: Insert the fusion gene fragment (GM-CSF)-scFv(meso)-CD8-CD28-CD137-CD3ζ into the lentiviral expression vector pLent-C-GFP.
[0050] Anti-meso's CAR module comments figure 1 (See appendix SEQ ID NO.1 for the complete nucleic acid sequence).
[0051] Anti-meso's CAR module sequence
[0052] (1) GM-CSF signal peptide nucleic acid artificial sequence (SEQ ID NO.2)
[0053] (2) Anti-mesothelin monoclonal antibody single chain Fv antibody (scFv) nucleic acid artificial sequence (SEQ ID NO.3)
[0054] (3) CD8αHinge region nucleic acid artificial sequence (SEQ ID NO.4)
[0055] (4) CD28 transmembrane region and intracellular region nucleic acid artificial sequence (SEQ ID NO.5)
[0056] (5) CD137 intracellular nucleic acid artificial sequence (SEQ ID NO.6)
[0057] (6) CD3ζ intracellular region nucleic acid artificial sequence (SEQ ID NO.7)
[0058] According to artificial nucleic acid sequence of signal peptide, artificial nucleic acid sequence of Anti-meso, art...
Embodiment 2
[0059] Example 2: Preparation of Chimeric Antigen Receptor (GM-CSF)-scFv(meso)-CD8-CD28-CD137-CD3ζ Modified T Cells
[0060] (1) Preparation of heterogeneous T cells—CIK
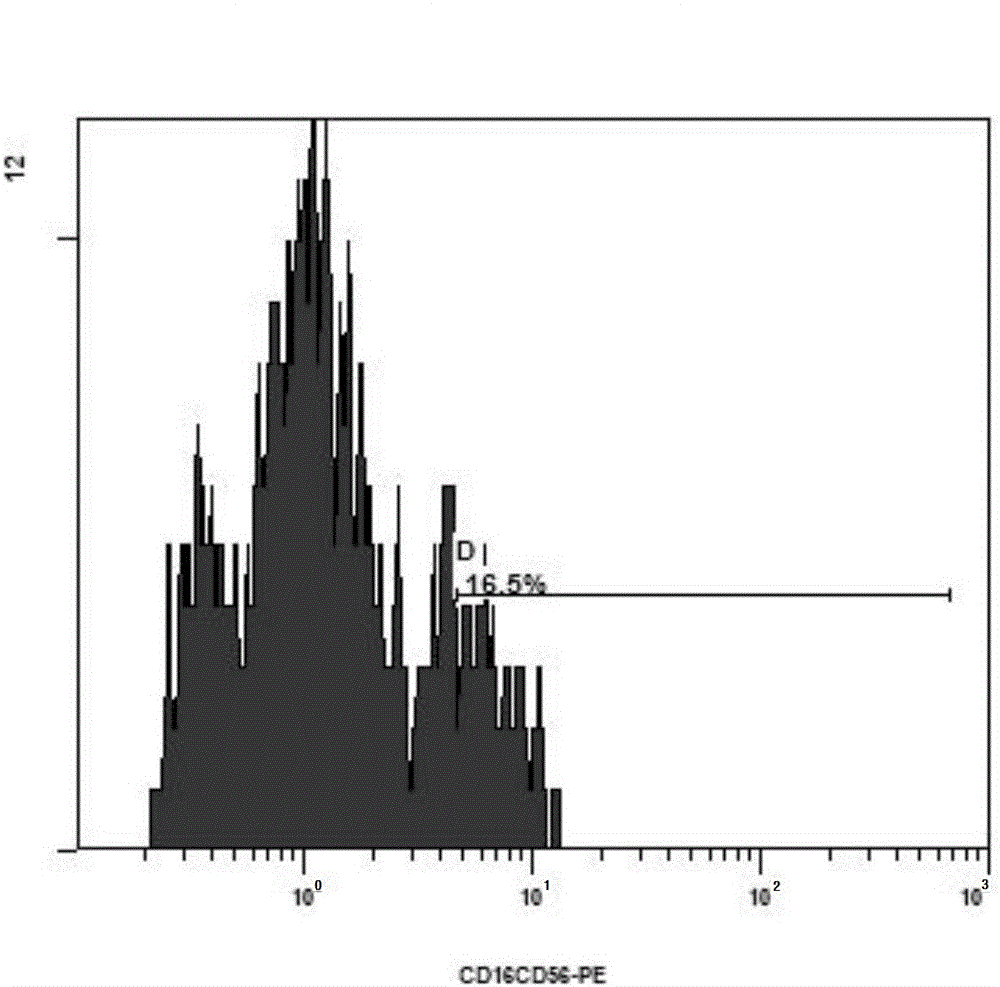
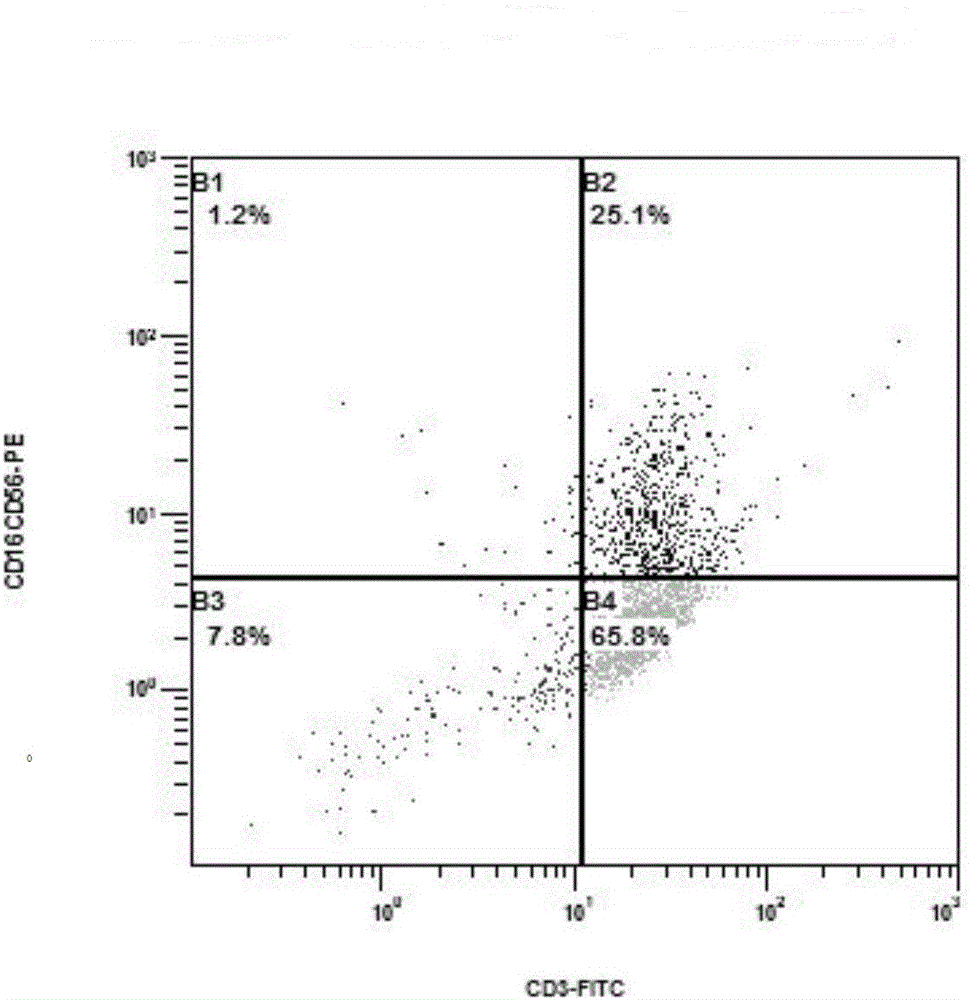
[0061] Take 75ml of autologous peripheral blood from the patient, and separate peripheral blood mononuclear cells with TBD sample density separation medium (purchased from Tianjin Haoyang Huake Biology). After inducing culture for 24 hours with a culture medium (purchased from CORNING Company, 88-551-CM) containing 1000 IU / ml of recombinant interferon α2a (purchased from Shenyang Sansheng Pharmaceutical), 1000 IU / ml of recombinant interleukin 2 ( (purchased from Shenyang Sansheng Pharmaceutical), 50ng / ml of OKT-3 and 5% of the patient's autologous plasma were induced and continued to be cultured for 24 hours. Doubling liquid was added every three days, cultured until the 14th day, and the positive expression rate of CD3+ and CD56+ in CIK cells was detected by flow cytometry (CD3-FITC, CD16 / CD56-PE antibodie...
Embodiment 5
[0069] Example 5: Determination of in vitro killing effect of T cell lines after genetic modification
[0070] According to different effect-to-target ratios (50:1, 25:1, 5:1, 1:1), modified cells and unmodified T cells were co-cultured with A431, A431-H9, HAY, and LDH lactate dehydrogenase was used - Cytotoxicity Assay Kit (LDH-Cytotoxicity Assay Kit, Biovision) detects the ability of genetically modified T cells to kill different types of tumor cells in vitro. The method is as follows: Target cells were plated in a 96-well plate (5×10 3 / well), set medium background, volume correction, target cell spontaneous LDH release, target cell maximum LDH release, effector cell spontaneous LDH release control well, treatment group wells, each group repeated 3 wells, the final volume of each well was the same and Not less than 100 μL. Centrifuge at 250g for 4min, and incubate at 37°C, 5% CO2 for at least 4h. 45 min before centrifugation, add 10× lysate to the target cell maximum rel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com