External release testing method of liposome medicaments prepared by pH gradient active drug loading method
An active drug-loading method and testing method technology, which is applied in the testing field of liposome drugs, can solve problems such as the inability to simulate the in vivo and in vitro release relationship of drugs, the prolonged main release period of doxorubicin, and the indistinct details of the release curve. , to achieve enhanced differentiation, high reliability, and easy implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Rotation - 52°C - 10% Resin - 33mM Ammonium Chloride
[0062] Steps A and B are the same as Comparative Examples 1 and 2.
[0063] C. In vitro release test
[0064] a. Add 5ml doxorubicin liposome dilution in the EPPENDORF centrifuge tube of 5ml;
[0065] b, adding ammonium chloride in the solution to form a mixed solution, so that the concentration of ammonium chloride is 33mM;
[0066] c. Add 0.5g (w / v=10%) cation exchange resin to the mixed solution;
[0067] d. Set the temperature of the molecular hybridization instrument at 52° C. and the rotation speed at 0 rpm, and add samples while preheating;
[0068] e. After sealing the centrifuge tube, fix it on the hybridization tube rack of the molecular hybridization instrument perpendicular to the rotation axis in a radial and centripetal manner;
[0069] f. Set the temperature of the molecular hybridization instrument at 52° C., and the rotational speed at 25 rpm, start to rotate and count.
[0070] g. T...
Embodiment 2
[0084] Example 2: 52°C - 10% resin - 48mM ammonium chloride
[0085] Steps A, B, and C are all the same as in Example 1. The difference is that the final concentration of ammonium chloride in the mixed solution is 48mM.
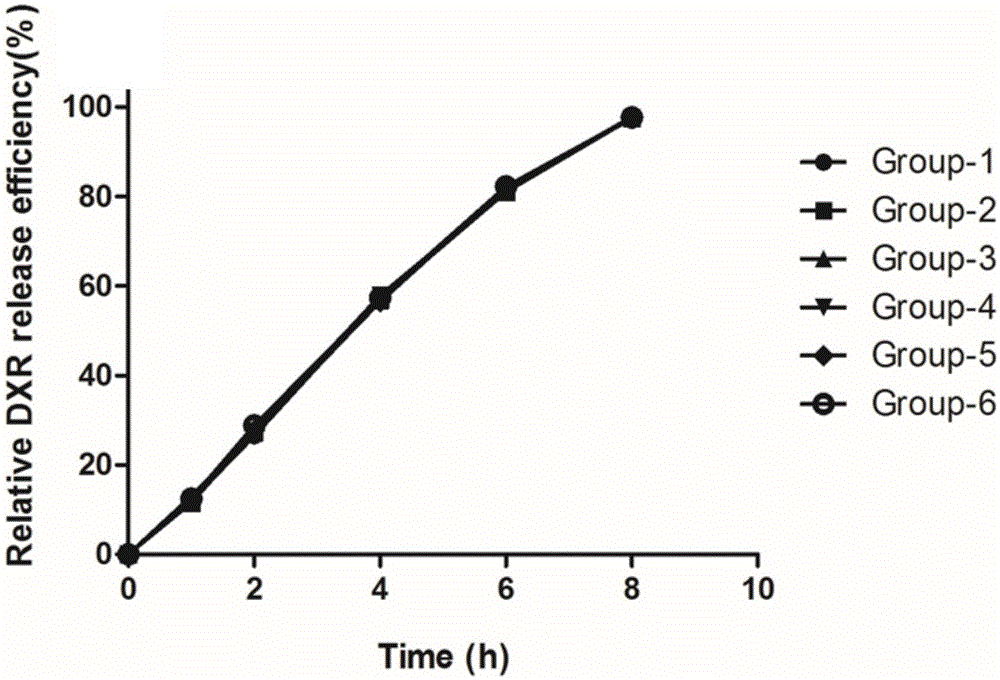
[0086] Samples were taken at the time points of 1h, 2h, 4h, 6h, and 8h, and 6 samples were taken at each time point, and the standard deviation (SD) was calculated. The test results are shown in Table 2.
[0087] 6 samples release rate of each time point in table 2, embodiment 2
[0088]
[0089] attached figure 2 It is a plot of the data in Table 2. It can be seen from the figure that the release rate of doxorubicin is very close in the 6 groups of investigations operated at the same time, and the repeatability of the method is very good.
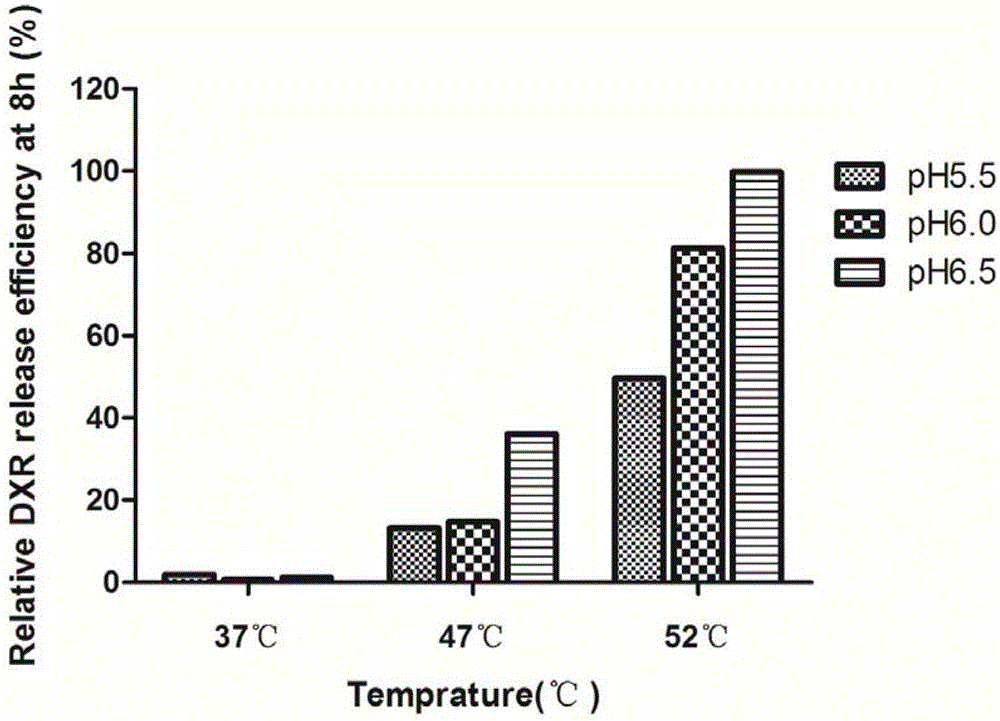
[0090] (3) Investigate the influence of temperature and the pH value of PBS buffer on the release rate
Embodiment 3
[0091] Example 3: 37°C-10% resin-33mM ammonium chloride-pH5.5
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com