Preparation method of high-temperature proton exchange membrane material and application of material
A proton exchange membrane, high temperature technology, applied in the field of clean energy, can solve the problem that the loading rate cannot meet the design accuracy and limitations of materials, and achieve the effects of superior electrical conductivity, low cost and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of the high temperature proton exchange membrane material (BIL25) of 25% loading capacity
[0032]1) Weigh 0.166g (1.0mmol) of terephthalic acid, 0.400g (1.0mmol) of chromium nitrate nonahydrate as a solid in the reactor lining of 15mL polytetrafluoroethylene, and then add a concentration of 40wt% by weight to it. After 0.05mL of HF solution and 7mL of deionized water, the reactor was filled, and the reaction kettle was placed in a blast drying oven heated to 220°C, and reacted for 8h. After the reaction was over, the reactor was taken out from the drying oven and cooled to room temperature naturally. The obtained dark green reaction mixture was transferred into a 10mL centrifuge tube, and centrifuged for 5min at a speed of 5500rmp / min. After centrifugation, the supernatant was discarded, and the obtained dark green solid was washed 3 to 5 times with methanol to wash away unreacted reactants and other impurities in the reaction mixture.
...
Embodiment 2
[0040] Embodiment 2: the preparation of the high temperature proton exchange membrane material (BIL50) of 50% loading capacity
[0041] Except for using 76 μl of ZIL / HTFSA ionic liquid, follow the same steps in Example 1 to prepare a 50% by weight loaded high-temperature proton exchange membrane material, that is, a composite material of ZIL / HTFSA and MIL-101.
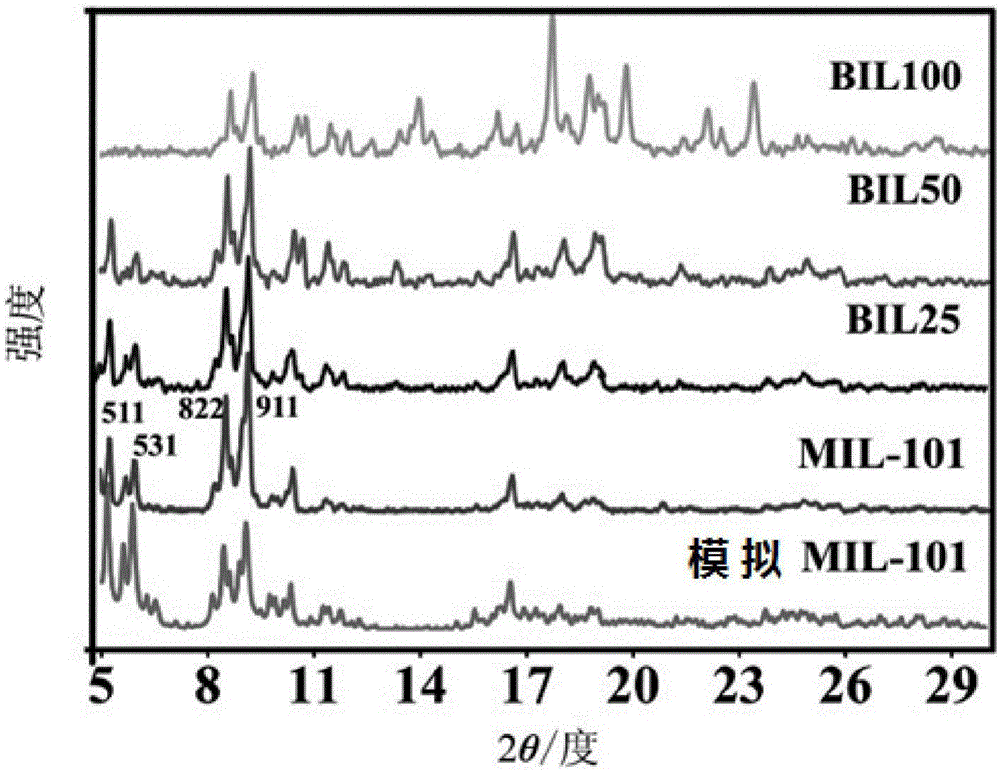
[0042] figure 1 The BIL50 curve in the middle is the X-ray powder diffraction pattern of the high-temperature proton exchange membrane material obtained according to this embodiment.
Embodiment 3
[0043] Embodiment 3: the preparation of the high temperature proton exchange membrane material (BIL100) of 100% loading capacity
[0044] Except for using 152 μl of ZIL / HTFSA ionic liquid, follow the same steps in Example 1 to prepare a 50% by weight loaded high-temperature proton exchange membrane material, that is, a composite material of ZIL / HTFSA and MIL-101.
[0045] figure 1 The BIL100 curve in the middle is the X-ray powder diffraction pattern of the high-temperature proton exchange membrane material obtained according to this embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com