Method for qualitative and quantitative analysis of triazophos and kit used in the method
A technology of triazophos and a kit, which is applied in the field of food safety detection and immunoassay, can solve problems such as inapplicability of triazophos, and achieve the effects of ensuring sensitivity, improving sensitivity, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation method of triazophos biological barcode immunoassay assay kit
[0050] Preparation of S11 and triazophos double-labeled colloidal gold probes
[0051] Gold Nanoparticle Synthesis Method
[0052] Add 98mL of deionized water to the siliconized beaker respectively, and then add 2mL of 50mM chloroauric acid stock solution respectively, so that the concentration of chloroauric acid is 1mM, stir on a magnetic stirrer at 1000rpm / min, heat at 250°C, continue to boil after the liquid boils Boil for 2 minutes; absorb 10mL of 38.8mM trisodium citrate, quickly add it to the beaker at one time, keep the stirring speed and heating temperature constant, and continue to boil for 6 minutes. It can be found that the color of the solution gradually changes from colorless to purple, and then into wine red. According to the different reducing doses of trisodium citrate added, it finally turns into different colors. After the liquid is cooled, deionized water is used to restore ...
Embodiment 2
[0076] Kit use method of the present invention
[0077] The specific operations of the triazophos colloidal gold composite probe prepared in the above implementation case 1 combined with the real-time quantitative PCR immunoassay assay kit are as follows:
[0078] S21. Take out the kit from the refrigerator at 4°C, and equilibrate at room temperature for 10 minutes;
[0079]S22, add each concentration triazophos standard substance (10.0ng L -1 -40 μg L -1 ) and 20 μL of each sample obtained after pretreatment, set up a repetition, then add 20 μL of magnetic nanoprobes to each well, fully oscillate and mix with a micro shaker, and then place the temperature at 37 ° C and a relative humidity of 70% constant temperature Incubate for 1 h in a constant humidity incubator;
[0080] S23, wash three times with PBST solution, dehybridize to obtain the biological barcode;
[0081] S24, put into fluorescent quantitative PCR to measure;
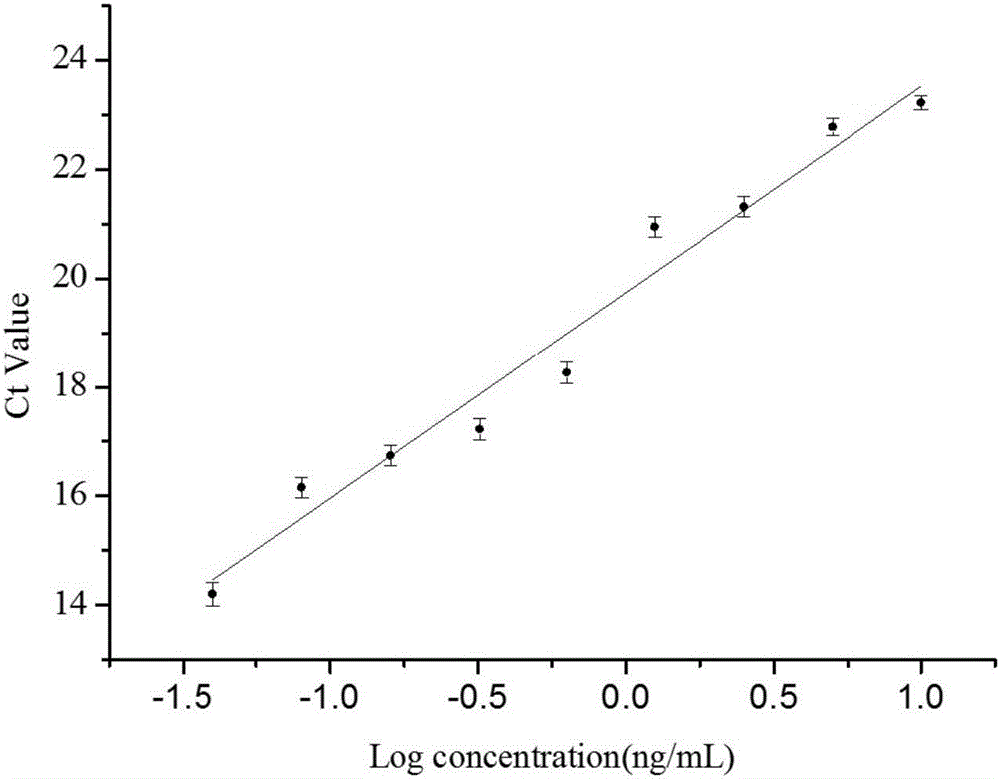
[0082] S25. Taking the concentration of the s...
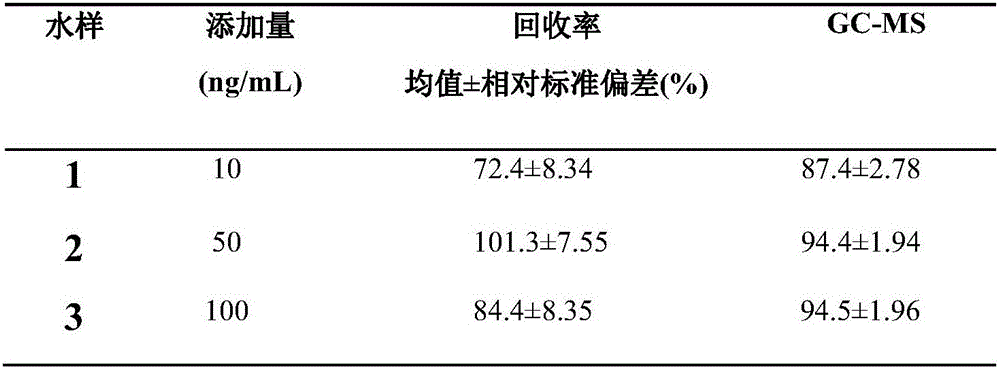
experiment example 1
[0084] The methodological examination result of kit of the present invention
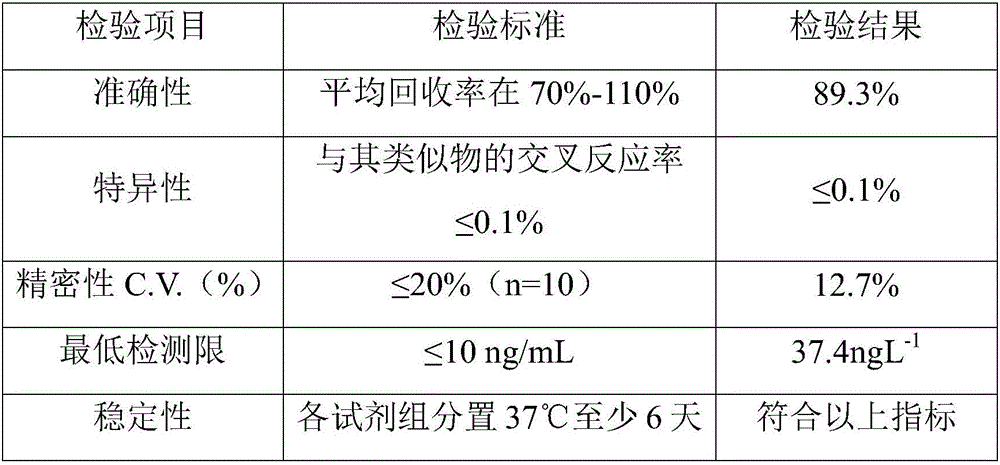
[0085] The kit prepared in Example 1 was identified according to the common verification procedures in this field, and the results are shown in Table 1.
[0086] The methodological testing result of test kit of the present invention of table 1
[0087]
[0088] The above results show that the accuracy, specificity, precision, sensitivity and stability of the "triazophos colloidal gold composite probe combined with real-time quantitative PCR immunoassay assay kit" are fully in line with the conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com