Application of PGD2 (prostaglandin D2) in preparation of drug for treating stomach cancer
A prostaglandin and drug technology, applied in the field of tumor treatment drugs, can solve problems affecting the expansion and differentiation of tumor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
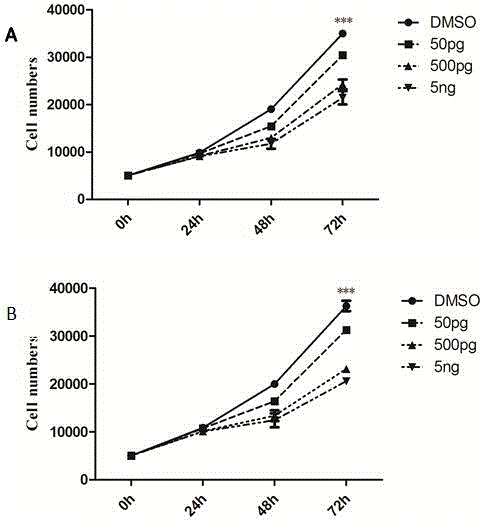
[0047] Example 1: Exogenous PGD2 inhibits the in vitro expansion and migration of gastric cancer cell lines SGC7901 and HGC-27
[0048] (1) Dilution of pure PGD2: Purchase PGD2 factor (1 mg) from Sigma, USA, add 100 μL of dimethyl sulfoxide (DMSO) reagent to dissolve, dilute to a mother solution with a concentration of 100 ng / μL, and then use blank 1640DMEM For the culture solution, dilute the mother solution at a ratio of 1:1000 to 100pg / μL nutrient solution (all the application solutions mentioned in the subsequent steps are at this concentration);
[0049] (2) The effect of exogenous PGD2 on the proliferation of tumor cells in vitro: HGC-27 and SGC-7901 cells were planted in 24-well plates with a cell density of 5000 / well, and different amounts of PGD2 application solutions were added to stimulate the cells. The final concentrations of exposure were: 50pg / mL, 500pg / mL and 5ng / mL, DMSO was used as the control. After 24h, 48h, and 72h of different concentrations of PGD2, the...
Embodiment 2
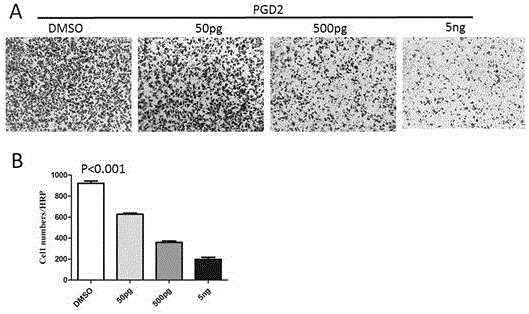
[0051] Example 2: Exogenous PGD2 inhibits gastric cancer cell self-renewal ability and related gene expression
[0052] The dilution of PGD2 pure product is with embodiment 1.
[0053] (1) Inhibitory effect of exogenous PGD2 on tumor cell sphere formation ability (self-renewal ability): first prepare stem cell serum-free medium (formulation: 20ng / mL EGF, 10ng / mL FGF, 1:50 B27 factor), HGC-27 and SGC-7901 cells were planted in 6-well plates, the culture condition was serum-free stem cell culture medium, and the cell density was 5000 / well. At the same time, different amounts of PGD2 application solution were added for stimulation, so that the final concentration of cells contacted was : 50pg / mL, 500pg / mL and 5ng / mL, DMSO was used as the control, after 7 days of culture, the number of spheres was observed under a 40-fold microscope (a sphere with a diameter greater than 70μm), and the size of the spheres was observed under a 200-fold microscope. It was shown that, compared with ...
Embodiment 3
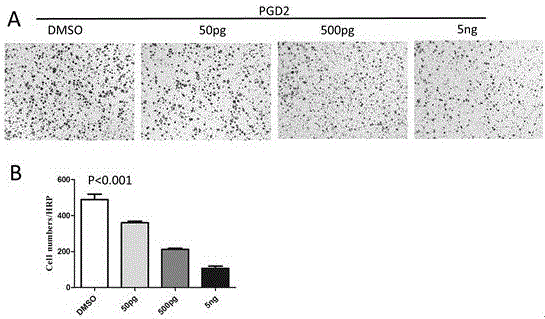
[0057] Example 3: Knocking down the rate-limiting enzyme L-PTGDS and PGD2 receptor molecule CRTH2 synthesized by PGD2 in gastric cancer by small hairpin RNA (shRNA) proved the inhibitory effect of endogenous PGD2 on the proliferation and migration of tumor cells in vitro, and suggested that This role is accomplished through CRTH2 signaling.
[0058] The key molecules involved in Example 3 are introduced: L-PTGDS is the key enzyme for the synthesis of PGD2. In other words, the expression level of L-PTGDS affects the amount of PGD2 secreted by the cells themselves. Knocking down the expression of L-PTGDS can effectively reduce PGD2 CRTH2 is an important receptor for PGD2 binding, mainly expressed on the cell membrane, and is an important molecule for PGD2 to transmit signals into cells. Knocking down CRTH2 can block the function of PGD2 on target cells.
[0059] The specific implementation steps of Embodiment 3 of the present invention are described as follows:
[0060] (1) Con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com