A kind of isomerization and hydroformylation reaction method and catalyst of internal olefin
A catalyst and isomerization technology, applied in the direction of organic compound/hydride/coordination complex catalyst, carbon monoxide reaction preparation, physical/chemical process catalyst, etc. The effect of low n-iso and high n-aldehyde and iso-aldehyde ratio and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
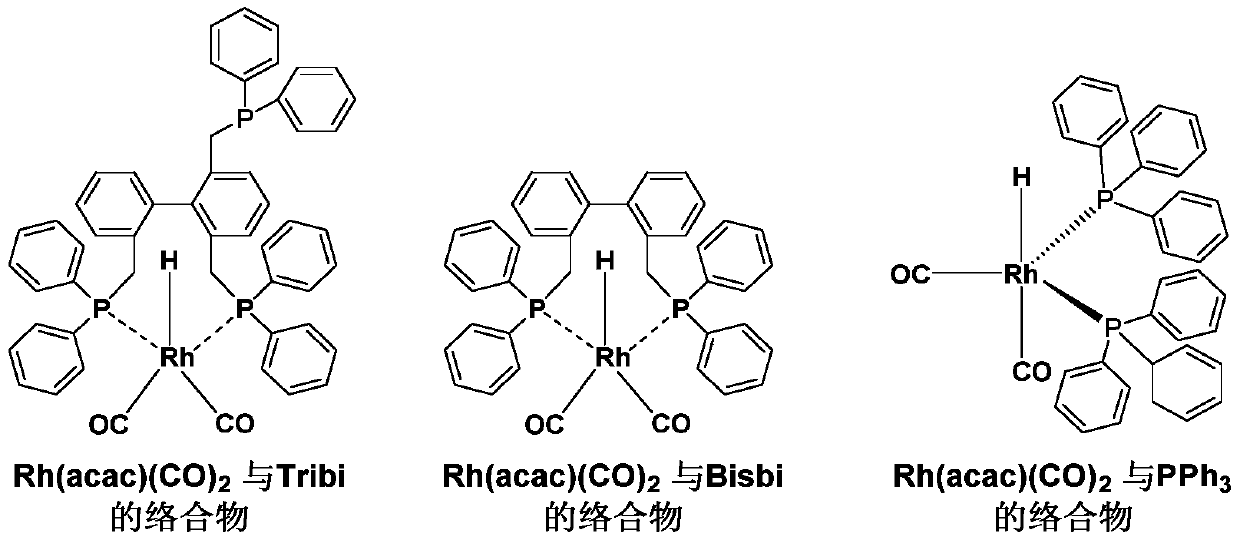
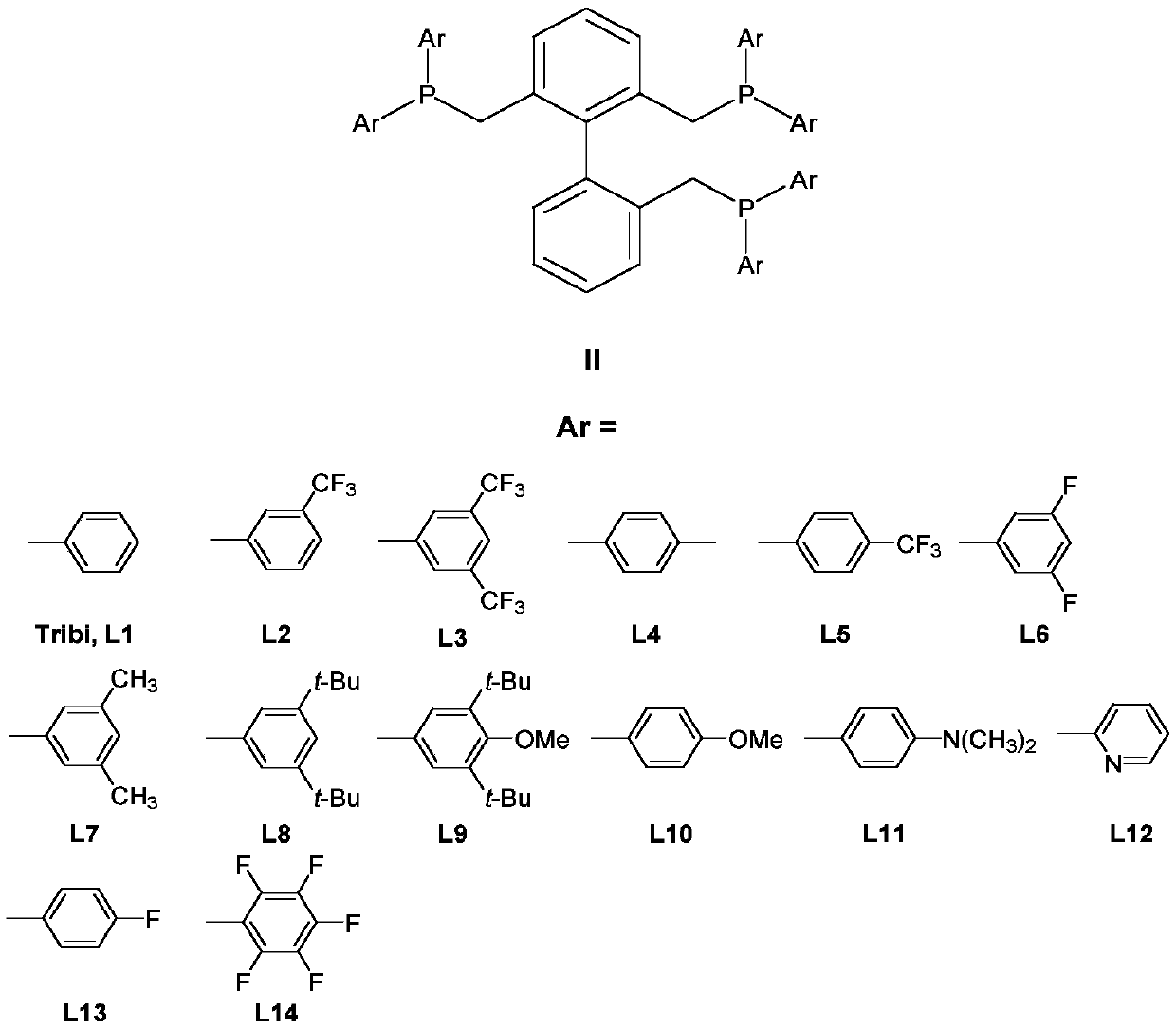
[0033] Embodiment 1: adopt rhodium acetylacetonate dicarbonyl (Rh(acac)(CO) 2 ) and 2,2,6-tris(diphenylphosphinemethyl)-1,1’-biphenyl (Tribi) hydroformylation (different phosphorus-rhodium ratio L / Rh)
[0034] The rhodium catalyst Rh(acac)(CO) was weighed in the glove box according to the different molar ratios of phosphorus ligand to rhodium listed in Table 1 below. 2 (2.6mg, 0.01mmol), 2,2,6-tris(diphenylphosphinemethyl)-1,1'-biphenyl (Tribi) (7.5mg, 0.01mmol; 15mg, 0.02mmol; 22mg, 0.03mmol ; 30mg, 0.04mmol; 45mg, 0.06mmol) into the complexing bottle, then put the toluene (Toluene, 1.73g, 18.8mmol) solvent of deoxygenation / water into the bottle, stir to make it dissolve to form rhodium and biphenyl triphenyl Complex solutions of phosphorus ligands. Put the autoclave into the glove box, pipette 200 μl of the complexed rhodium catalyst solution into the reaction bottle (5ml) with a magnet, add 100 μl of internal standard n-decane and 400 μl of toluene solvent , and finally ...
Embodiment 2
[0039] Embodiment 2: adopt rhodium acetylacetonate dicarbonyl (Rh(acac)(CO) 2 ) and 2,2,6-tris(diphenylphosphinomethyl)-1,1’-biphenyl (Tribi) hydroformylation (different reaction temperatures)
[0040] The rhodium catalyst Rh(acac)(CO) was weighed in a glove box according to the molar ratio of phosphorus ligand to rhodium (4:1) listed in Table 2 below. 2 (2.6mg, 0.01mmol), 2,2,6-tris(diphenylphosphinemethyl)-1,1'-biphenyl (Tribi) (30mg, 0.04mmol) into the complexation bottle, and then deoxygenated / water toluene (Toluene, 1.73g, 18.8mmol) solvent was placed in the bottle, and stirred to dissolve it to form a complex solution of rhodium and biphenyl triphosphorus ligand. Put the autoclave into the glove box, pipette 200 μl of the complexed rhodium catalyst solution into the reaction bottle (5ml) with a magnet, add 100 μl of internal standard n-decane and 400 μl of toluene solvent , and finally 2-octene (cis-trans mixture) (224.4 mg, 2 mmol) was added. Subsequently, the react...
Embodiment 3
[0044] Embodiment 3: adopt rhodium acetylacetonate dicarbonyl (Rh(acac)(CO) 2 ) and 2,2,6-tris(diphenylphosphinomethyl)-1,1'-biphenyl (Tribi) hydroformylation reaction (different reaction pressure, S / C=2000, S / C is the reaction molar ratio of catalyst to catalyst)
[0045] According to example 2, the rhodium catalyst complex solution of the same concentration is prepared in the glove box, the autoclave is put into the glove box, and the rhodium catalyst solution that 200 μ l is complexed is pipetted to the reaction that the magneton is placed In the bottle (5ml), add 100μl internal standard n-decane and 400μl toluene solvent, and finally add 2-octene (cis-trans mixture) (224.4mg, 2mmol). Subsequently, the reaction kettle with the reaction bottle was taken out from the glove box, and was washed with H 2 Replace the high-purity argon in the kettle 3 times with CO / H 2Raise the total pressure of the reactor to 4 bar, 5 bar, 10 bar, 20 bar and 40 bar at a pressure ratio of 1:1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com