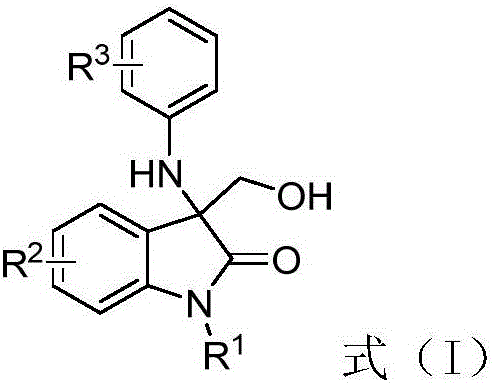
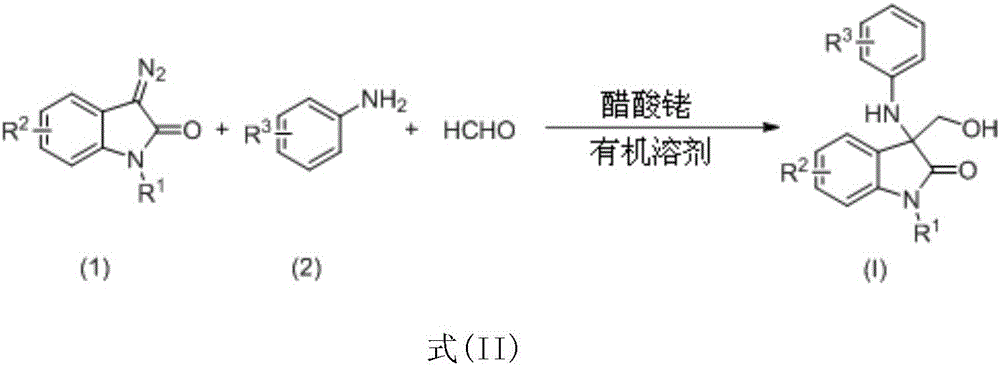
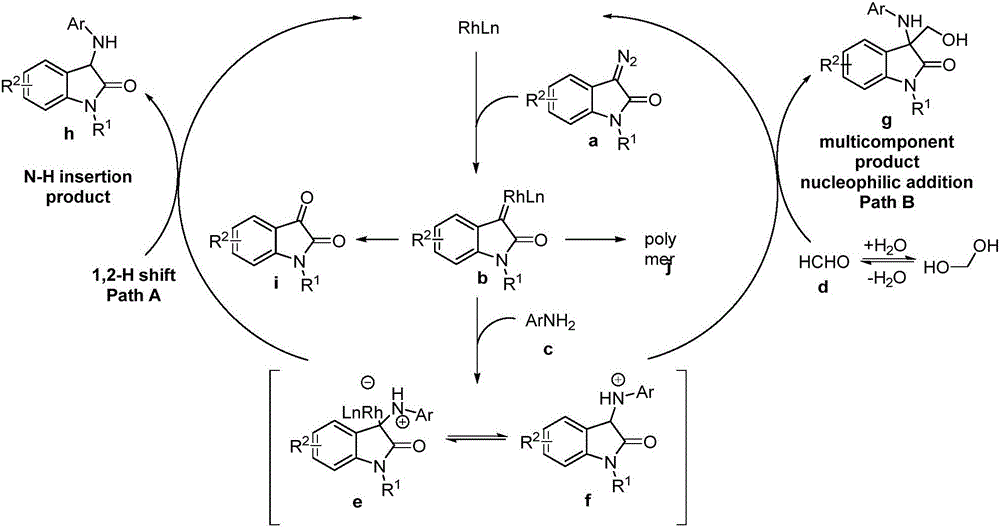
3-amino-3-hydroxymethyloxindole derivative as well as preparation method and application thereof
A technology of indole derivatives, indole diazonium oxide, applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of special structure, harsh reaction conditions, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 prepares compound Ia of the present invention
[0055] Dissolve 2,6-dichloroaniline (0.1mmol), formaldehyde (0.6mmol) and rhodium acetate (0.001mmol) in 1mL ethyl acetate to form a reaction system, keep the temperature at 60°C, and use an automatic injection pump to inject 1-formaldehyde A solution of 3-diazo-2-oxindole (0.1 mmol) dissolved in 1 mL of ethyl acetate was added to the reaction system in 1 hour. After the injection was completed, the reaction was continued to stir at 60° C. for 1 hour. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, which was purified by column chromatography (petroleum ether: ethyl acetate = 10:1-1:1) to obtain pure product Ia. Yield: 78%. See Table 1.
[0056]
[0057] Characterization of 3-amino-3-hydroxymethyloxindole derivative Ia:
[0058] 1 H NMR (400MHz, CDCl 3 )δ7.26(d, J=6.5Hz, 1H), 7.11(d, J=8.0Hz, 2H), 6.86–6.73(m, 3H), 6.68(d, J=7.3Hz, 1H), 5.22( s, 1H), 3.9...
Embodiment 2-22
[0060] Embodiment 2-22 prepares compound (Ib~Iv)
[0061] Embodiment 2-22 preparation process is the same as embodiment 1. See Table 1 for the changes of substituents, compound numbers, yields, etc. during the reaction.
[0062] Table 1
[0063]
[0064]
[0065] The characterization of product 3-amino-3-hydroxymethyloxindole derivatives Ib~IV is as follows:
[0066] Characterization of Ib:
[0067] 1 H NMR (400MHz, CDCl 3 )δ7.40–7.27(m,5H),7.15(td,J=7.8,1.0Hz,1H),7.09(d,J=8.0Hz,2H),6.78(dt,J=12.4,4.1Hz,3H ),6.69–6.59(m,1H),5.27(s,1H),5.04(d,J=15.4Hz,1H),4.82(d,J=15.4Hz,1H),3.97(t,J=10.9Hz ,1H),3.87(dd,J=11.3,3.0Hz,1H),3.15(dd,J=10.4,3.1Hz,1H).
[0068] 13 C NMR (100MHz, CDCl 3 )δ178.1, 143.0, 140.0, 135.5, 129.7, 129.4, 128.8, 128.1, 127.9, 127.8, 125.8, 124.1, 123.7, 122.3, 109.6, 69.8, 66.5, 44.1.
[0069] Characterization of Ic:
[0070] 1 H NMR (400MHz, CDCl 3 )δ7.39–7.27(m, 6H), 7.14(d, J=8.0Hz, 2H), 6.83(t, J=8.0Hz, 1H), 6.78(d, J=1.8Hz, 1H), 6.64( d...
Embodiment 23
[0129] Example 23 3-Amino-3-Hydroxymethyloxindole Derivatives Effects on Human Osteosarcoma Cells (SJSA-1), Human Colorectal Cancer Cells (HCT-116), Human Liver Cancer Cells (BEL7402), Human Oral Epidermoid Carcinoma Cells (KB) and human T-cell leukemia cells (Jurkat) the inhibitory effect on the proliferation of five tumor cells.
[0130] The present invention adopts CCK-8 method to measure the effect of 3-amino-3-hydroxymethyloxindole derivatives described in embodiment 1-22 (Ia-Iv) on human osteosarcoma cells (SJSA-1), human colorectal cancer Cells (HCT-116), human liver cancer cells (BEL7402), human oral epidermoid carcinoma cells (KB) and human T-cell leukemia cells (Jurkat) five tumor cell proliferation inhibitory effects.
[0131] Firstly, the activity of 22 compounds against the proliferation of SJSA-1 cells was determined, and then the compound with the best proliferation activity was selected, and its inhibitory activity against HCT-116 cells, BEL7402 cells, KB cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com