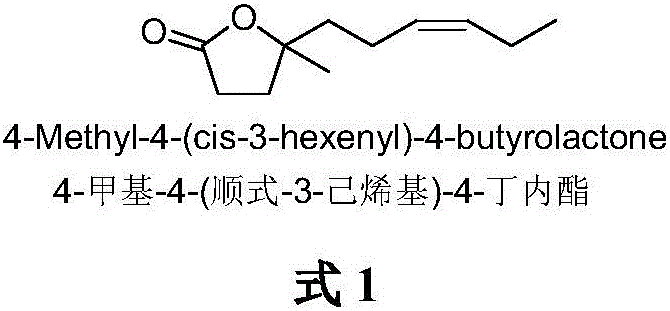
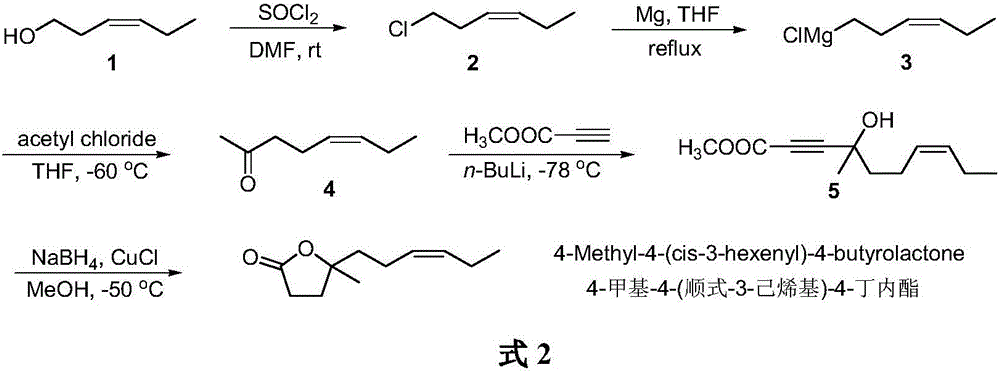
Synthetic method of 4-methyl-4-(cis-3-hexenyl)-4-butyrolactone
A synthesis method and hexenyl technology, applied in organic chemistry and other directions, can solve the problems of high cost, strong corrosiveness, and unsuitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Synthesis of cis 1-chloro-3-hexene
[0016] Add leaf alcohol (100.0g, 1.0mol) and DMF (154mL) into a 500mL three-necked flask, stir well, cool the mixture to 5-10°C, slowly add thionyl chloride (125.0g, 1.05mol) dropwise, control The temperature of the reaction solution is lower than 25°C. After the dropwise addition was completed, the reaction was stirred for another 3 h, and the gas chromatography detection showed that the leaf alcohol content of the raw material was less than 0.5%, and the reaction was stopped. Distilled under reduced pressure to remove the solvent and thionyl chloride. The residue was washed with deionized water until neutral, and dried over anhydrous sodium sulfate to obtain cis-1-chloro-3-hexene (109.0 g, yield 92%) as a colorless liquid. 1 H NMR (400MHz, CDCl 3 )δ5.56-5.49(m,1H),5.36-5.29(m,1H),3.49(t,2H),2.53-2.47(m,2H),2.09-2.01(m,2H),0.96(t, 3H).GC-MS: m / z (relative intensity) 118 (M + ,39),41(100),55(86),69(77).
[0017] (2) Synthes...
Embodiment 2
[0025] (1) Synthesis of cis 1-chloro-3-hexene
[0026] Add leaf alcohol (200.0g, 2.0mol) and DMF (300mL) into a 1000mL three-necked flask, stir well, cool the mixture to 5-10°C, slowly add thionyl chloride (250.0g, 2.1mol) dropwise, control The temperature of the reaction solution is lower than 25°C. After the dropwise addition was completed, the reaction was stirred for another 5 h. Gas chromatography detection showed that the leaf alcohol content of the raw material was less than 0.5%, and the reaction was stopped. Distilled under reduced pressure to remove the solvent and thionyl chloride. The residue was washed with deionized water until neutral, and dried over anhydrous sodium sulfate to obtain cis-1-chloro-3-hexene (210.0 g, yield 89%) as a colorless oil. Product of cis-1-chloro-3-hexene 1 H NMR and GC-MS are the same as in Example 1.
[0027] (2) Synthesis of cis-5-hexen-2-one
[0028] Add THF (480mL) and magnesium chips (52.0g, 2.2mol) into a 1000mL three-necked f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com