Brexpiprazole crystal, its preparation method and application, and pharmaceutical composition comprising brexpiprazole crystal
A technology of epirazole and a composition, applied in the field of medicinal chemistry, can solve the problems such as few crystal forms of epirazole, and achieve the effects of low cost, good stability and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of the crystalline form of Epirizopril:
[0036] Put 500.0mg of epirazole powder in a test tube, slowly add isopropanol at 80°C until it just completely dissolves (approximately 12mL of isopropanol is added), cool slowly to 20°C, gradually crystallize, pump Filter and air-dry at 85±5°C for 4 hours to obtain a white powder. Table 1 shows the X-ray powder diffraction data of the crystal form of the sample obtained in this embodiment.
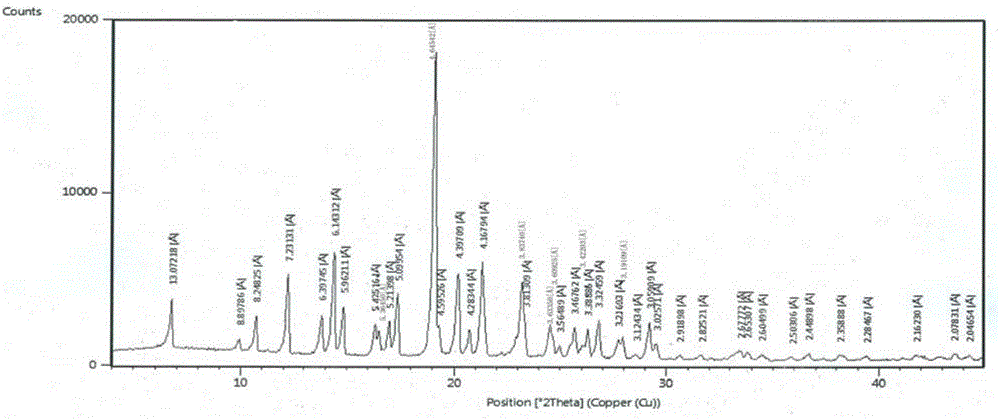
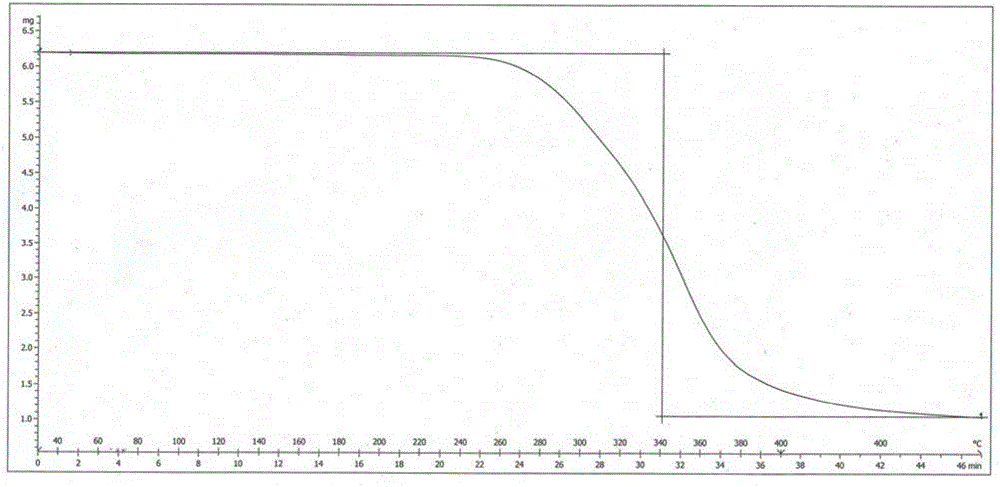
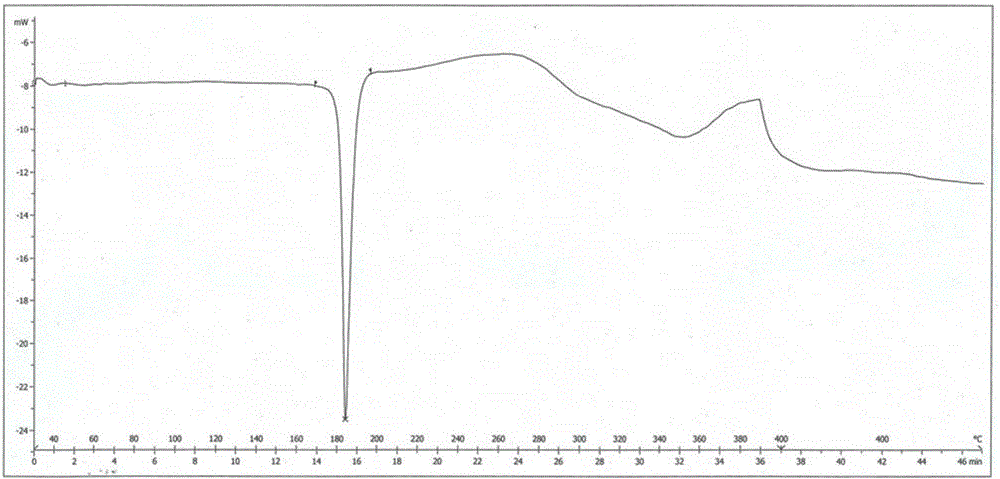
[0037] The X-ray powder diffraction pattern (XRPD pattern) of crystal form is as figure 1 Shown; The crystal form begins to show an endothermic peak when it is heated to 184.3 ° C, and its differential scanning calorimetry (DSC chart) is shown in figure 2 Shown; When the crystal form is heated to 340 ° C, it has a weight loss gradient, and its thermogravimetric analysis diagram (TGA diagram) is as follows image 3 shown.
[0038] The X-ray powder diffraction data of table 1 epizopra crystal form
[0039]
[0...
Embodiment 2
[0042] The preparation method of the crystalline form of Epirizopril:
[0043] Put 500.0 mg of Epirizopam powder in a test tube, add 10 ml of tetrahydrofuran at 65°C, dissolve, slowly cool to 0°C, gradually crystallize, filter with suction, and air-dry at 85±5°C for 5 hours to obtain white powder.
Embodiment 3
[0045] The preparation method of the crystalline form of Epirizopril:
[0046] Put 500.0mg of Epirizopam powder in a test tube, slowly add 25mL of ethyl acetate at 78°C, dissolve it, cool slowly to -10°C, gradually crystallize, filter with suction, and air-dry at 85±5°C for 4h A white powder was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com