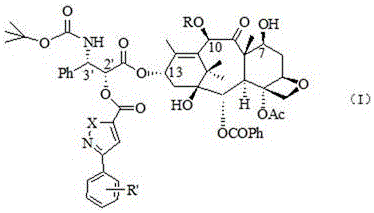
Docetaxel side chain 2'-derived novel taxanes antitumor compound as well as synthesis method and application thereof
A compound and natural product technology, applied in the direction of antineoplastic drugs, organic chemistry, drug combination, etc., can solve the problems of poor water solubility and limited wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
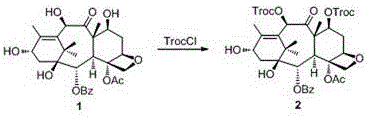
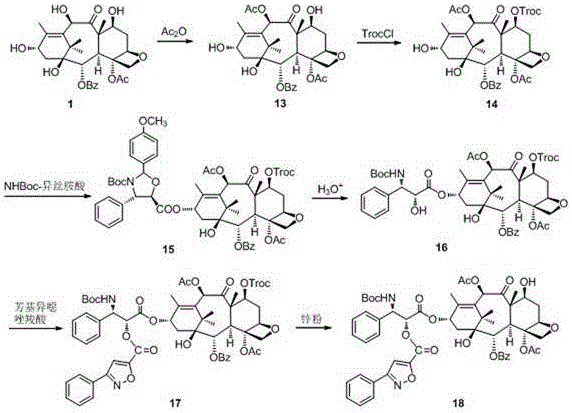
[0036] Example 1 Synthesis of 7,10-diTroc-baccatin 2
[0037] Slowly add 20.5 mmol of trichloroethoxycarbonyl chloride (TrocCl) dropwise to 10-DAB (10 mmol) dissolved in pyridine at 63-95°C, stir magnetically, and monitor with TLC (petroleum ether-AcOEt) After the reaction was complete, the reaction solution was cooled to room temperature and pyridine was removed under reduced pressure, and the residue was washed with AcOEt / H 2 After O was dissolved and diluted, the pH of the solution was adjusted to neutral with HCl. The solution was left to stand for stratification, the organic phase was separated, the aqueous phase was extracted with AcOEt, the organic phases were combined and successively washed with H 2 O, washed with saturated brine, anhydrous MgSO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product, and 7,10-diTroc-baccatin 2 was obtained by column chromatography, the yield: 87.4%, m.p. 233.1-234.2°C. 1 H NMR (400 MHz, CDCl 3 ) δ...
Embodiment 2
[0038] Example 2 Synthesis of 10-Ac-baccatin 13
[0039] Add 0.2 mol of acetic anhydride to 10 mmol of 10-DAB and ZnCl dissolved in 80 mL of anhydrous THF 2 20 mmol solution, reacted at room temperature, and added AcOEt / H 2 O with paste NaHCO 3 Adjust the pH of the solution to alkaline. Stand to separate the layers, separate the organic phase, extract the aqueous phase with AcOEt, combine the organic phases and successively use H 2 O, washed with saturated brine, anhydrous MgSO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product. After recrystallization, the pure product of 10-Ac-baccatin 13 was obtained as a white solid, with a yield of 93%. 1 H NMR (400 MHz, CDCl 3 ) δ 8.12 (d, J = 7.7 Hz, 2H), 7.63 (t, J =7.3 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 6.34 (s, 1H), 5.64 (d, J = 6.9 Hz,1H), 5.00 (d, J = 9.1 Hz, 1H), 4.90 (t, J = 7.9 Hz, 1H), 4.48 (dd, J = 10.5,6.8 Hz, 1H), 4.32 (d, J = 8.4 Hz, 1H), 4.17 (d, J = 8.3 Hz, 1H)...
Embodiment 3
[0040] Example 3 Synthesis of 7-Troc-10-Ac-baccatin 14
[0041] At 50-93°C, slowly add 9.8 mmol of trichloroethoxycarbonyl chloride (TrocCl) dropwise to compound 13 (9.3 mmol) dissolved in pyridine, stir magnetically, monitor the completion of the reaction by TLC, and cool the reaction solution to room temperature and remove pyridine, the residue was treated with AcOEt / H 2 After O was dissolved and diluted, the pH of the solution was adjusted to be slightly alkaline with HCl. The solution was left to stand for stratification, the organic phase was separated, the aqueous phase was extracted with AcOEt, the organic phases were combined and successively washed with H 2 O, washed with saturated brine, anhydrous MgSO 4 After drying, the solvent was removed to obtain a crude product, which was subjected to column chromatography to obtain 7-Troc-10-Ac-baccatin 12 with a yield of 84.2%. m.p.154.8-158.2°C. 1 H NMR (400MHz, CDCl 3 ) δ 8.11 (d, J = 8.1 Hz, 2H), 7.63 (t, J = 7.4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com