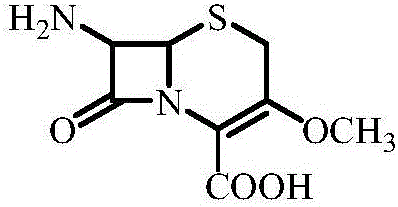
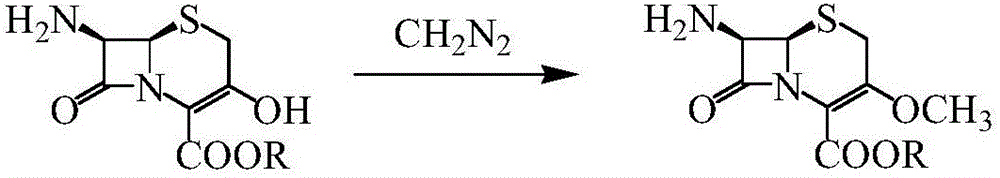
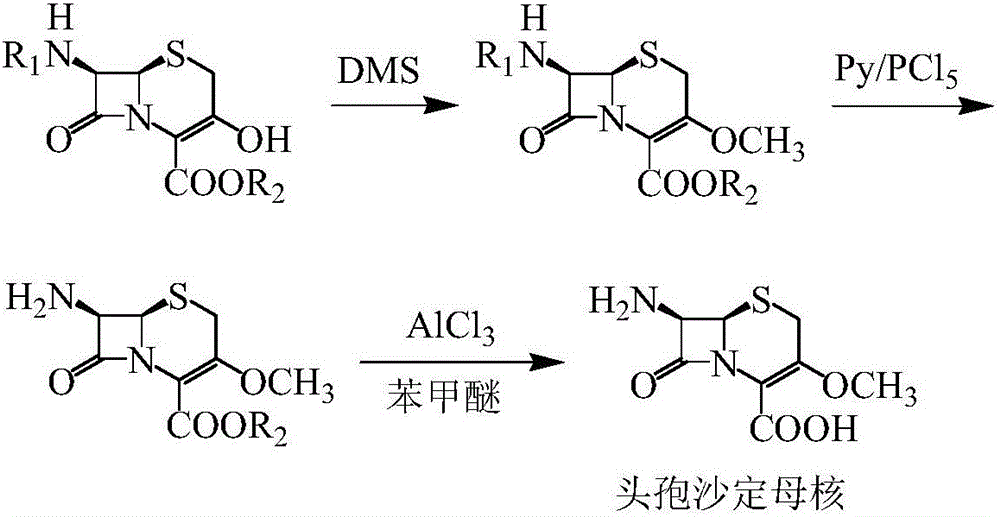
Method for synthesizing parent nucleus of cefroxadine
A technology of ceftoxadine and a synthesis method, which is applied in the field of synthesis of ceftoxadine parent nucleus, can solve problems such as unfavorable safety production, metal aluminum solid waste, unfavorable safety management, etc., and achieves environmental protection, good quality, and operation The effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of BH:
[0031] Add 3-hydroxycephalosporin (50.06g, 0.10mol), methanol (100mL), CHCl 3 (200mL), HC(OMe) 3 (11.67g, 0.11mol), stirred to dissolve, added anhydrous p-toluenesulfonic acid (TsOH) (0.86g, 0.005mol) and stirred to reflux for 8h, sampling, HPLC detection of 3-hydroxycephalosporin residual less than 1%, the reaction was completed. Cool down to 0-5°C, add 50mL of pure water, stir for 10min, separate the layers, and wash the water layer with CHCl 3 Extract twice, 50 mL each time, combine the organic layers, add 5 g of anhydrous magnesium sulfate and stir for 10 min, then add 5 g of activated carbon and stir for 10 min, filter with suction, and collect the filtrate.
[0032] Control the internal temperature of the filtrate to less than 30°C and distill to dryness under reduced pressure, add CH 2 Cl 2 (300mL), cooled to -10~-5℃, added pyridine (11.87g, 0.15mol), CBr 4 (36.48g, 0.11mol), triphenylphosphine (28.85g, 0.11mol), temperature controlled at ...
Embodiment 2
[0036] Synthesis of BH:
[0037] Add 3-hydroxycephalosporin (50.06g, 0.10mol), methanol (150mL), CHCl 3 (150mL), HC(OMe) 3 (10.61g, 0.10mol), stirred and dissolved, added anhydrous p-toluenesulfonic acid (TsOH) (0.86g, 0.005mol) and stirred and refluxed for 8h, sampling, HPLC detection of 3-hydroxycephalosporin residual less than 1%, the reaction was completed. Cool down to 0-5°C, add 50mL of pure water, stir for 10min, separate the layers, and wash the water layer with CHCl 3 (50 mL / time) extracted twice, combined the organic layers, added 5 g of anhydrous magnesium sulfate and stirred for 10 min, then added 5 g of activated carbon and stirred for 10 min, filtered with suction, and collected the filtrate.
[0038] Control the internal temperature of the filtrate to less than 30°C and distill to dryness under reduced pressure, add CH2 Cl 2 (300mL), cooled to -10~-5℃, added pyridine (7.91g, 0.10mol), CBr 4 (33.16g, 0.10mol), triphenylphosphine (26.23g, 0.10mol), temperature...
Embodiment 3
[0042] Synthesis of BH:
[0043] Add 3-hydroxycephalosporin (50.06g, 0.10mol), methanol (75mL), CHCl 3 (225mL), HC(OMe) 3 (12.73g, 0.12mol), stirred and dissolved, added anhydrous p-toluenesulfonic acid (TsOH) (0.86g, 0.005mol) and stirred and refluxed for 8h, sampling, HPLC detection of 3-hydroxycephalosporin residual less than 1%, the reaction was completed. Cool down to 0-5°C, add 50mL of pure water, stir for 10min, separate the layers, and wash the water layer with CHCl 3 (50 mL / time) extracted twice, combined the organic layers, added 5 g of anhydrous magnesium sulfate and stirred for 10 min, then added 5 g of activated carbon and stirred for 10 min, filtered with suction, and collected the filtrate.
[0044] Control the internal temperature of the filtrate to less than 30°C and distill to dryness under reduced pressure, add CH 2 Cl 2 (300mL), cooled to -10~-5℃, added pyridine (15.83g, 0.20mol), CBr 4 (39.80g, 0.12mol), triphenylphosphine (31.47g, 0.12mol), temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com