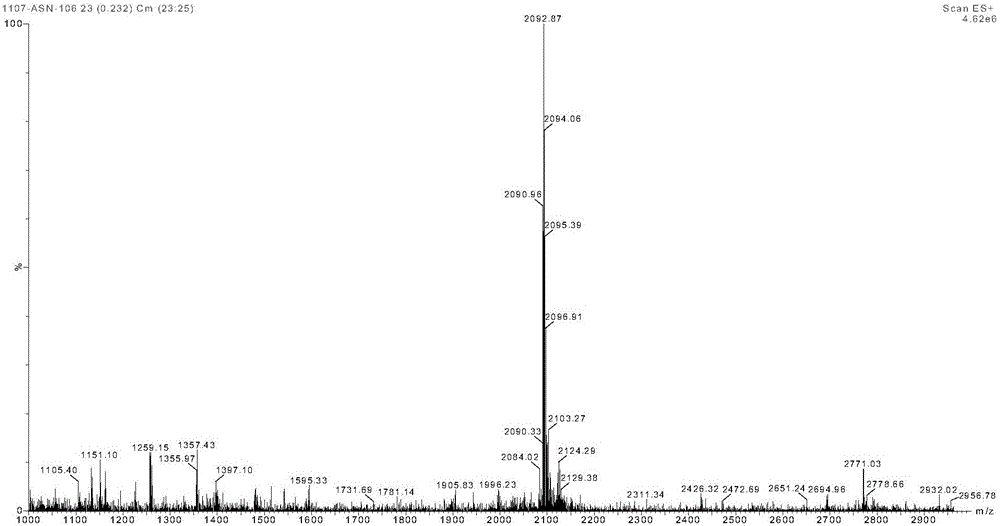
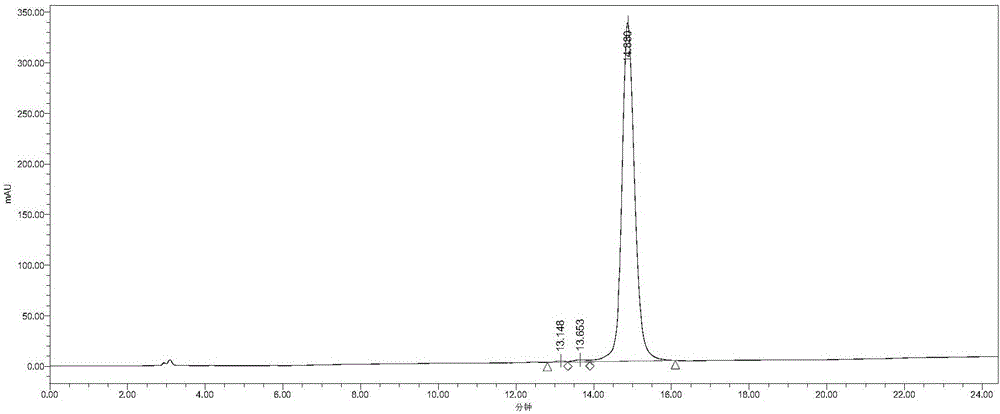
Method for preparing exenatide and product thereof
A technology for exenatide and products, applied in the field of exenatide synthesis by fragment method, which can solve the problems of low product content, high purification cost, and low efficiency of synthesizing long peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The present invention provides a kind of preparation method of exenatide, described method passes Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro -Ser-CONH 2 and the fully protected fragments of the following three fragments were prepared: Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-COOH, Thr-Ser-Asp-Leu-Ser-Lys-Gln-COOH, His-Gly-Glu-Gly-Thr-Phe-COOH. This is the result of the inventor analyzing the sequence and combining the properties of each amino acid, taking into account the cost of synthesis, choosing between various factors and experimenting, and splitting the positions of 39 amino acids into the above-mentioned Four fragments. The above selection is the result of the inventor's creative work, which effectively reduces the cost;
[0088] Wherein the fully protected fragments are respectively Fmoc-Phe-Ile-Glu(Otbu)-Trp-Leu-Lys(Boc)-Asn(Trt)-Gly-Gly-Pro-Ser(tBu)-Ser(tBu)-Gly -Ala-Pro-Pro-Pro-Ser(tBu)-Rink AmideMBHA Resin, Fmoc-Met-Glu(Otbu)-Glu(Otbu)-Gl...
Embodiment 1
[0130] 1. Synthesis of Fmoc-Phe-Ile-Glu(Otbu)-Trp-Leu-Lys(Boc)-Asn(Trt)-Gly-Gly-Pro-Ser(tBu)-Ser(tBu)-Gly-Ala-Pro- Pro-Pro-Ser(tBu)-Rink Amide MBHA Resin
[0131] (1) Synthesis of Fmoc-Ser(tBu)-MBHA Resin
[0132] Add 50g of Fmoc-Rink Amide MBHA Resin into the reactor, add 500mL N,N-dimethylformamide to soak the resin for 30 minutes to fully swell the resin, remove N,N-dimethylformamide by suction filtration, and pour into the reactor Add 500mL piperidine and N,N-dimethylformamide with a volume ratio of 1:4 mixture, react for 5 minutes, remove the mixture by suction filtration, then add 500mL piperidine and N,N-dimethylformamide The mixed solution with a volume ratio of 1:4 was reacted for 20 minutes, suction filtered, and the resin was washed twice with isopropanol and three times with N,N-dimethylformamide, 500 mL each time, to complete the Fmoc-Rink Amide MBHA Resin de-Fmoc- twice, add 500mL N,N-dimethylformamide, 13.04g Fmoc-Ser(tBu)-OH, 4.59g 1-hydroxybenzotriazole, 12....
Embodiment 2
[0158] In step 1 of this implementation, Fmoc-Ser(tBu)-OH, Fmoc-Pro-OH, Fmoc-Pro-OH, Fmoc-Pro-OH, Fmoc-Ala-OH, Fmoc-Gly-OH, Fmoc-Ser( tBu)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Pro-OH, Fmoc-Gly-OH, Fmoc-Gly-OH, Fmoc-Asn(Trt)-OH, Fmoc-Lys(Boc)-OH, The molar ratio of Fmoc-Leu-OH, Fmoc-Trp-OH, Fmoc-Glu(Otbu)-OH, Fmoc-Ile-OH, Fmoc-Phe-OH to Fmoc-Rink Amide MBHA Resin is 3:1, Fmoc- Moles of Rink Amide MBHA Resin and 1-hydroxybenzotriazole, benzotriazole-N,N,N′,N′-tetramethylurea tetrafluoroboric acid, N,N′-diisopropylethylamine Ratio is 1:3:3:3, other steps of this step are identical with embodiment 1.
[0159] In step 2 of this example, Fmoc-Arg(pbf)-OH, Fmoc-Val-OH, Fmoc-Ala-OH, Fmoc-Glu(Otbu)-OH, Fmoc-Glu(Otbu)-OH, Fmoc-Glu The molar ratio of (Otbu)-OH, Fmoc-Met-OH and 2-chlororitylchloride resin is 3:1, 2-chlororitylchloride resin and 1-hydroxybenzotriazole, benzotriazole-N,N,N' , The molar ratio of N′-tetramethylurea tetrafluoroboric acid to N,N′-diisopropylethylamine is 1:3:3:3, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com