Ankara virus strain FAdV-HB and preparation and application of inactivated vaccine of ankara virus strain FAdV-HB
A technology of Ankara virus and inactivated vaccine, applied in the field of animal vaccine preparation, can solve the problem of strong pathogenicity of chickens, and achieve the effect of preventing Ankara disease, good safety and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
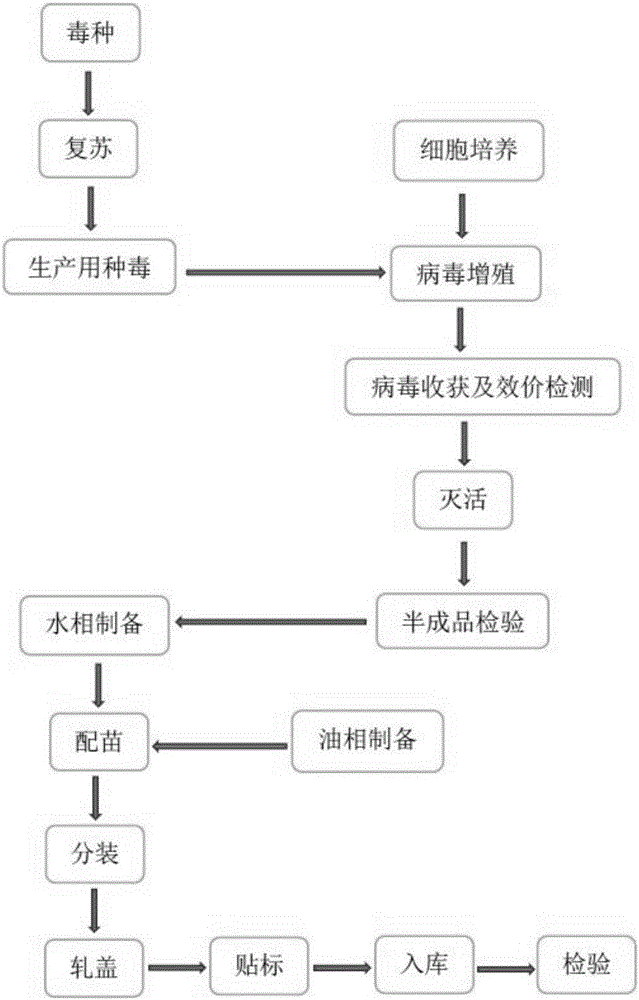
[0028] The preparation of embodiment 1 Ankara inactivated vaccine
[0029] 1. Isolation and identification of FAdV-HB strain
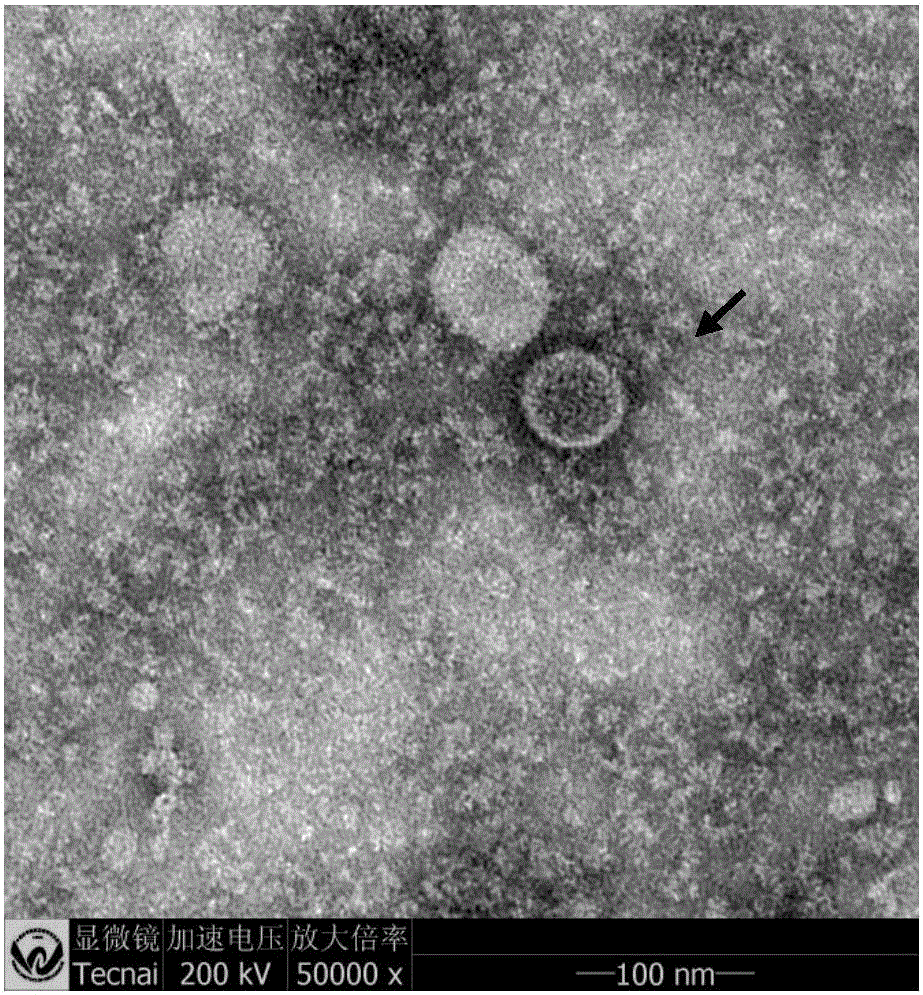
[0030] Since 2013, a poultry disease with a fatality rate of up to 80% has broken out in many broiler chicken farms in Hubei, Shandong, and Henan. Hepatitis symptoms. In 2015, the inventor successfully isolated the virus of this strain from the organs of dead chickens with severe hydropericardium and hepatitis in Macheng Chicken Farm, Hubei.
[0031] The specific operation steps are as follows:
[0032] 1.1 Virus isolation
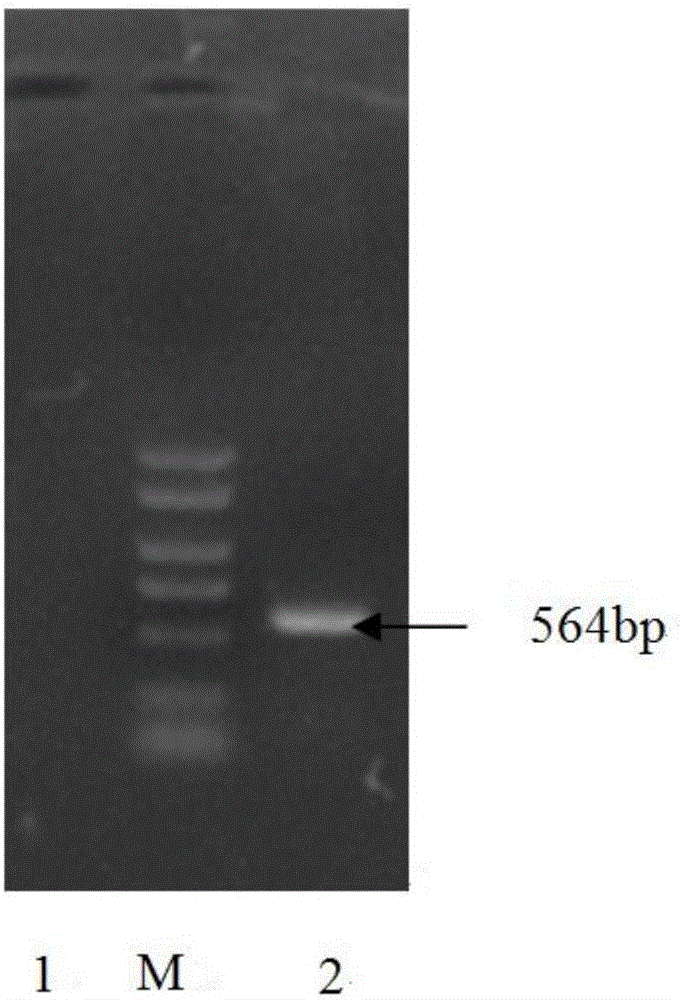
[0033] Aseptically collect viscera (heart, liver) with obvious lesions from sick and dead chickens, cut them into pieces and homogenate, add sterilized PBS at a ratio of 1:4 (v / v), freeze and thaw repeatedly 3 times, centrifuge at 3000r / min for 30min, and take Add penicillin and streptomycin to the supernatant so that the final concentration is 10000 units / ml, and overnight at 4°C. Use a 0.22 μm filter to filter and sterilize,...
Embodiment 2
[0201] Example 2 Safety Test of Ankara Virus Disease Live Vaccine
[0202] 1 Vaccines and experimental animals
[0203] The Ankara inactivated vaccines produced by the inventors were used, and the batch numbers were 0101, 0102, and 0103 respectively. The chicken breed used for the trial was Bailaihang, provided by Shandong Jinan Spifry Poultry Technology Co., Ltd.
[0204] 2 Safety trials of vaccines
[0205] This embodiment has detected the safety of above 3 batches of laboratory vaccines, and immunized Bailaihang chicks, and used to detect the safety of the batch of laboratory vaccines to Bailaihang chicks, including the safety of a single-dose inoculation of the vaccine to Bailaihang chicks, single Safety of repeated dose vaccination and safety of single overdose vaccination.
[0206] 2.1 The safety of a single-dose inoculation to Bailaihang chicks
[0207] After the three batches of vaccines were inoculated with 1-3 day-old chicks (Bai Laihang) in a single dose, the ch...
Embodiment 3
[0218] Example 3 Vaccine efficacy test of Ankara inactivated vaccine
[0219] 1 Ankara inactivated vaccine and experimental animals
[0220] 3 batches of Ankara inactivated vaccines produced by the inventor, the batch numbers are 0101, 0102, 0103 respectively. Ankara virus antibody-negative 1-3 day-old healthy chicks were used as experimental animals, provided by Jinan Spifry Poultry Technology Co., Ltd.
[0221] 2 Experimental methods
[0222] 40 1-3 day old healthy chicks were randomly divided into 4 groups, 10 in each group, three batches of vaccines prepared in the laboratory (batch numbers 0101, 0102, 0103) were immunized to one group in each batch, and the vaccine was injected intramuscularly into each leg 0.3ml. The remaining group was the non-immune control under the same conditions.
[0223] 3. Immunoprotective test
[0224] 3.1 Detection of antibody level
[0225] After 14 days of immunization, 30 chickens in the immune antibody monitoring group and 10 non-immu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com