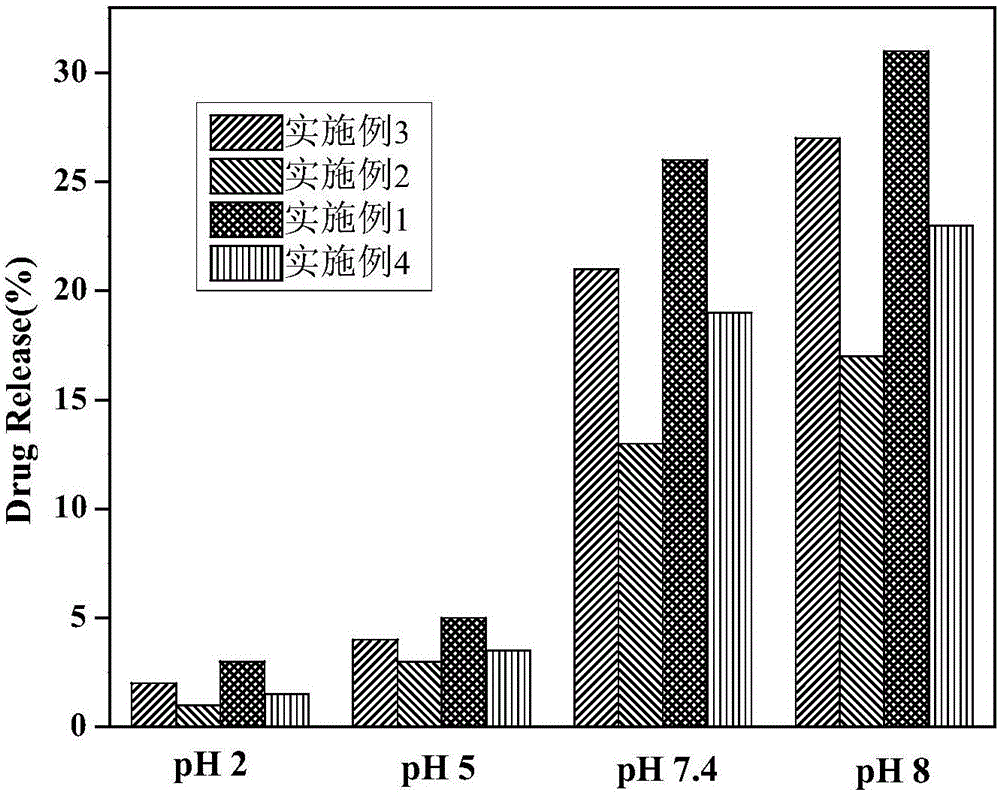
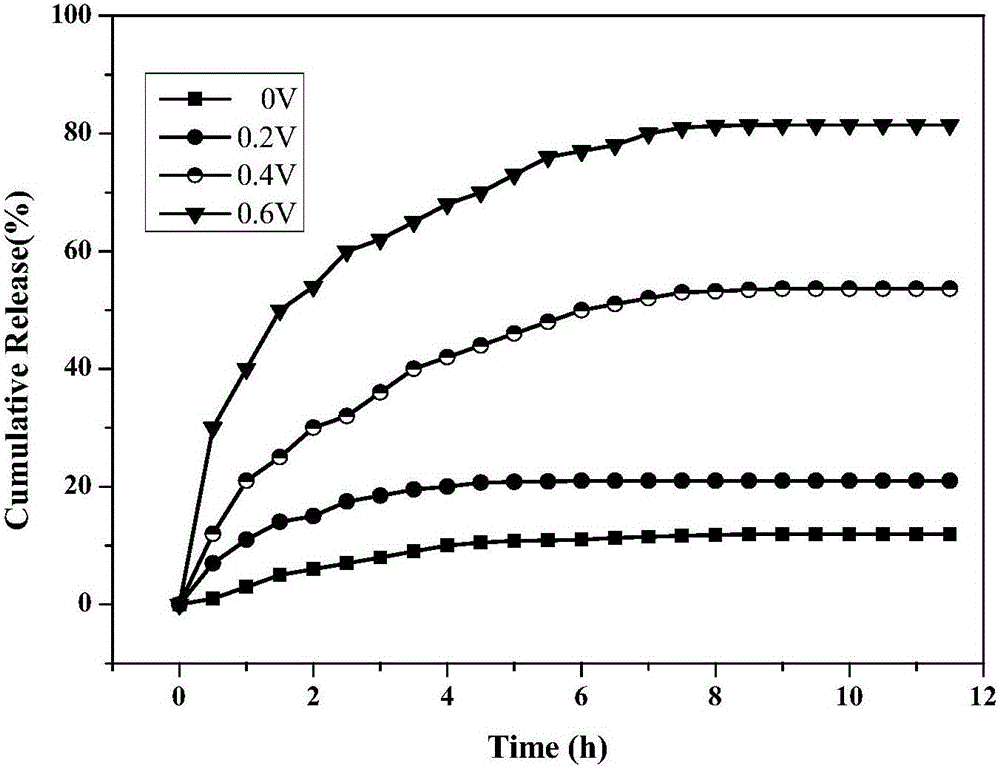
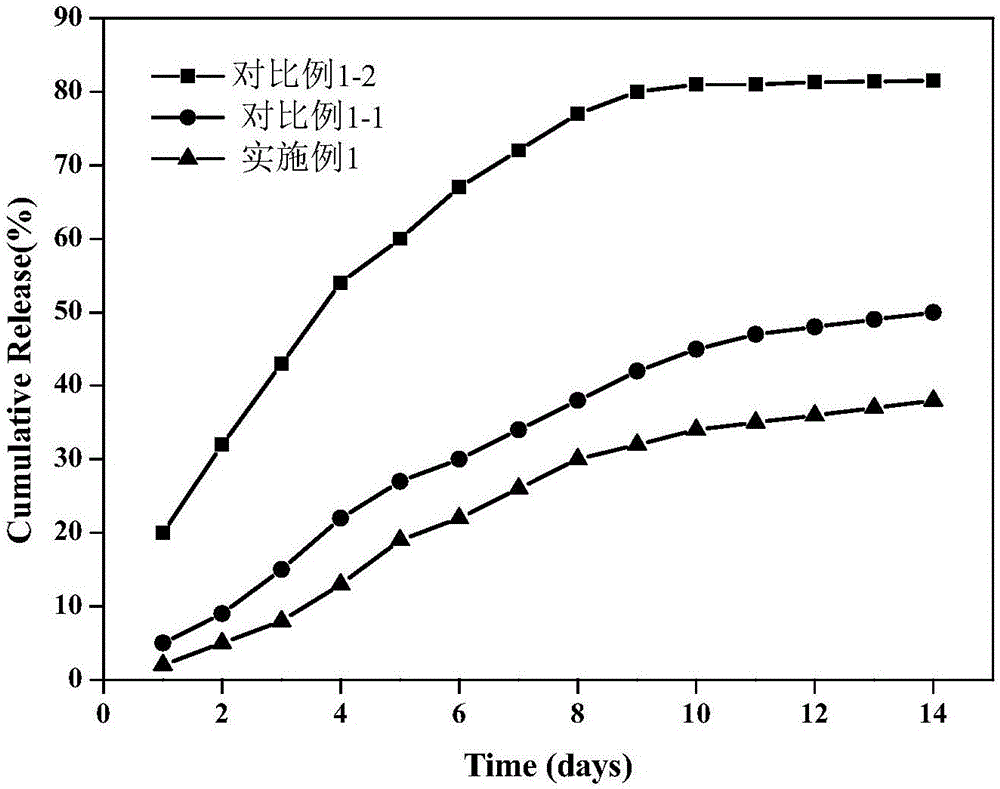
Preparation method for medicine-carrying polypyrrole/sodium alginate gel
A kind of technology of sodium alginate and polypyrrole, applied in the field of polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve 25mg of sodium alginate in 25mL of ionized water to prepare sodium alginate solution;
[0029] (2) Dissolve 0.4g pyrrole monomer in 50mL 1mol / L HCl solution to prepare pyrrole monomer solution;
[0030] (3) Dissolve 4mg of acetylsalicylic acid in 40mL of 1mol / L HCl solution to prepare a drug solution;
[0031] (4) Under magnetic stirring, drop the drug solution prepared in step (3) into the pyrrole monomer solution obtained in step (2), and stir for 15 minutes after the dropping;
[0032] (5) Under magnetic stirring, the drug-dissolved pyrrole monomer solution obtained in step (4) was added dropwise to the sodium alginate solution prepared in step (1), and after the addition was completed, ultrasonically dispersed for 15 minutes;
[0033] (6) Add 0.8g 30%H 2 o 2 Dissolve in 40mL deionized water, mix well; under ice bath condition, put H 2 o 2 The solution was slowly added dropwise to the mixed solution obtained in step (5). During the dropwise addition...
Embodiment 2
[0048] (1) Dissolve 75mg of sodium alginate in 25mL of ionized water to prepare sodium alginate solution;
[0049] (2) Dissolve 0.4g pyrrole monomer in 25mL 1mol / L HCl solution to prepare pyrrole monomer solution;
[0050] (3) Dissolve 20 mg of methotrexate in 40 mL of 1mol / L HCl solution to prepare a drug solution;
[0051] (4) Under magnetic stirring, drop the drug solution prepared in step (3) into the pyrrole monomer solution obtained in step (2), and stir for 30 minutes after the dropping;
[0052] (5) Under magnetic stirring, the drug-dissolved pyrrole monomer solution obtained in step (4) was added dropwise to the sodium alginate solution prepared in step (1), and after the addition was completed, ultrasonically dispersed for 25 minutes;
[0053] (6) 1.2g 30%H 2 o 2 Dissolve in 30mL deionized water, mix well; under ice bath condition, put H 2 o 2 The solution was slowly added dropwise to the mixed solution obtained in step (5). During the dropwise addition, magneti...
Embodiment 3
[0056] (1) Dissolve 50 mg of sodium alginate in 25 mL of ionized water to prepare a sodium alginate solution;
[0057] (2) Dissolve 0.4g pyrrole monomer in 40mL 1mol / L HCl solution to prepare pyrrole monomer solution;
[0058] (3) Dissolve 16 mg of dexamethasone in 40 mL of 1mol / L HCl solution to prepare a drug solution;
[0059] (4) Under magnetic stirring, drop the drug solution prepared in step (3) into the pyrrole monomer solution obtained in step (2), and stir for 20 minutes after the dropping;
[0060] (5) Under magnetic stirring, the drug-dissolved pyrrole monomer solution obtained in step (4) was added dropwise to the sodium alginate solution prepared in step (1), and after the addition was completed, ultrasonically dispersed for 20 minutes;
[0061] (6) Add 1.0g 30%H 2 o 2 Dissolve in 40mL deionized water, mix well; under ice bath condition, put H 2 o 2 The solution was slowly added dropwise to the mixed solution obtained in step (5). During the dropwise addition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com