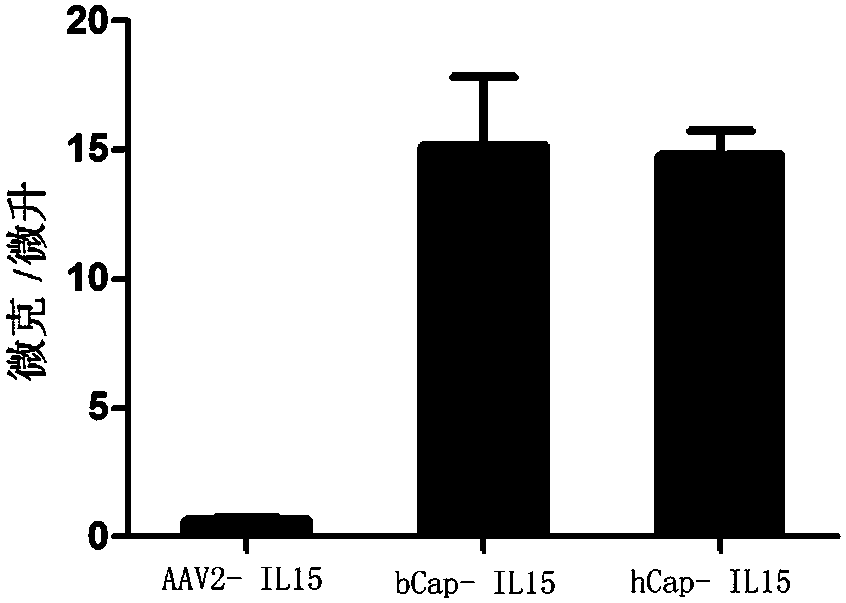
A kind of AAV virus capable of efficiently infecting immune cells and its preparation method and application
An immune cell and virus technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of low transduction efficiency, no AAV serotypes are found, and achieve low immunogenicity and human safety. , highly infectious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Directly synthesize the DNA sequence shown in SEQ ID NO:2 by chemical synthesis method, which is called hCap gene, and perform PacI-AscI double enzyme digestion to obtain the digested DNA fragment; use PacI-AscI double enzyme digestion pAAV-RC vector, recover the digested product, and obtain the digested vector; prepare the T4 ligation reaction system between the digested DNA fragment and the digested vector, and connect overnight at 16°C; thus clone the DNA sequence into the pAAV-RC vector, and After the sequence verification was correct, the ligated product (cloning product) was transformed into 30uL Stbl3 E. coli competent. Immediately after the transformation, add 1mL of preheated SOC medium, then transfer it to a 15mL centrifuge tube, and add 5mL of SOC medium, culture at 37 degrees 180RPM for 1 hour, transfer all the liquid to 800mL LB medium , adding ampicillin with a final concentration of 50ug / mL, and cultured in shake flasks at 37 degrees for 16 hours. Af...
Embodiment 2
[0032] (1) Directly synthesize the DNA sequence shown in SEQ ID NO:3 by chemical synthesis method, which is called bCap gene, and carry out PacI-AscI double enzyme digestion, clone it into pAAV-RC, after the sequence verification is correct, The ligated product was transformed into 30uL Stbl3 Escherichia coli competent by electroporation. Immediately after electroporation, add 1mL of preheated SOC medium, then transfer it to a 15mL centrifuge tube, add 5mL of SOC medium, culture at 37 degrees 180RPM for 5 hours, transfer all the liquid to 500mL LB medium Add ampicillin with a final concentration of 50ug / mL, and culture in shake flasks at 37 degrees for 18 hours. After the culture was over, the bacterial pellet was collected by centrifugation at 2000 g, and the plasmid DNA was isolated using a plasmid extraction kit, and the adeno-associated virus plasmid was obtained after sequencing to verify correctness. AAV virus that can efficiently infect immune cells.
Embodiment 3
[0034] (1) The hCap gene was directly synthesized by chemical synthesis, and subjected to PacI-AscI double enzyme digestion, and cloned into pAAV-RC, which was used for subsequent AAV virus packaging after being verified to be correct by sequencing. The ligated product was transformed into 30uL Stbl3 Escherichia coli competent by electroporation. Immediately after electroporation, add 1mL of preheated SOC medium, then transfer it to a 15mL centrifuge tube, add 5mL of SOC medium, culture at 37 degrees 180RPM for 5 hours, transfer all the liquid to 500mL LB medium Add ampicillin with a final concentration of 50ug / mL, and culture in shake flasks at 37 degrees for 18 hours. After the culture was over, the bacterial pellet was collected by centrifugation at 2000 g, and the plasmid DNA was isolated using a plasmid extraction kit, and the adeno-associated virus plasmid was obtained after sequencing to verify correctness.
[0035] (2) Prepare 20 15cm culture dishes and inoculate 4.5x...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com