Manufacture method for soluble microneedle
A manufacturing method and soluble technology, which can be applied in the direction of microneedles, skin care preparations, medical preparations with non-active ingredients, etc., and can solve problems such as limiting the application of soluble microneedles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
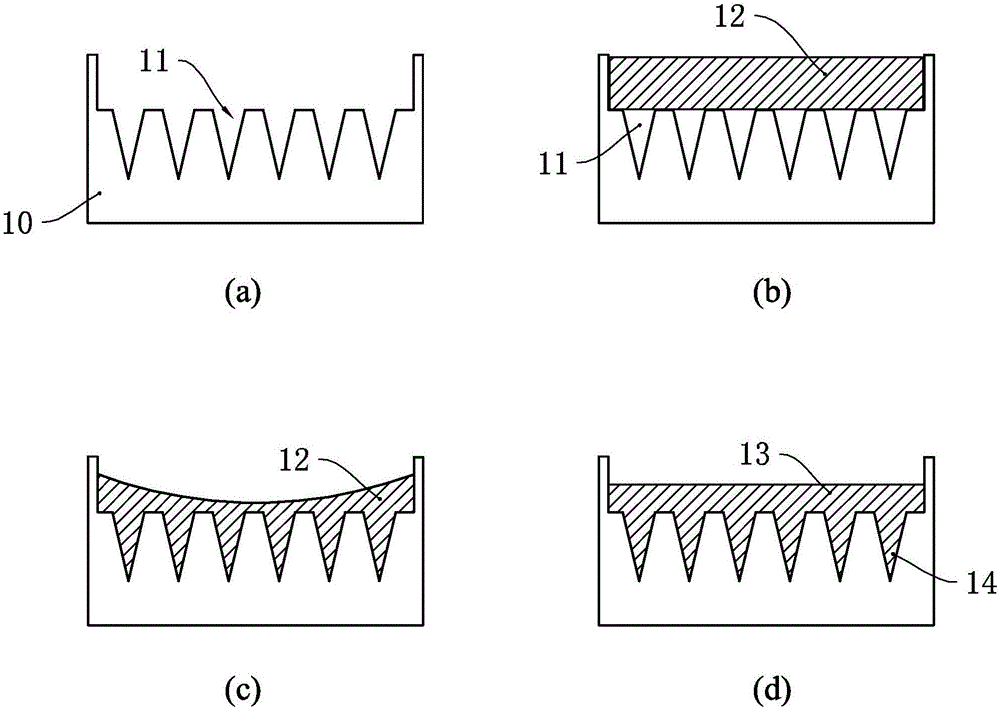
[0041] see figure 2 , in the microneedle manufacturing method of the present embodiment, the mold of the microneedle is first produced, the microneedle mold 20 has a plurality of micropore cavities 21, and each micropore cavity 21 is an inverted cone shape, of course, the shape of the micropore cavity It is not limited to the inverted tapered shape, and other shapes are also possible. Preferably, the height of each microneedle cavity 21 is between 50 microns and 1000 microns, and the inner diameter at the mouth of each microneedle cavity 21 is less than 1000 microns.
[0042] The microneedle mold 20 can be prepared using a wet etching process, for example, the pyramidal silicon microneedles are arranged in a 10×10 array, the length of the microneedle array is 200 microns, and the base diameter is 100 microns, or the cones The silicon microneedles are arranged in a 20×20 array. The length of the microneedle array is 800 microns, and the base diameter is 300 microns. needle c...
no. 2 example
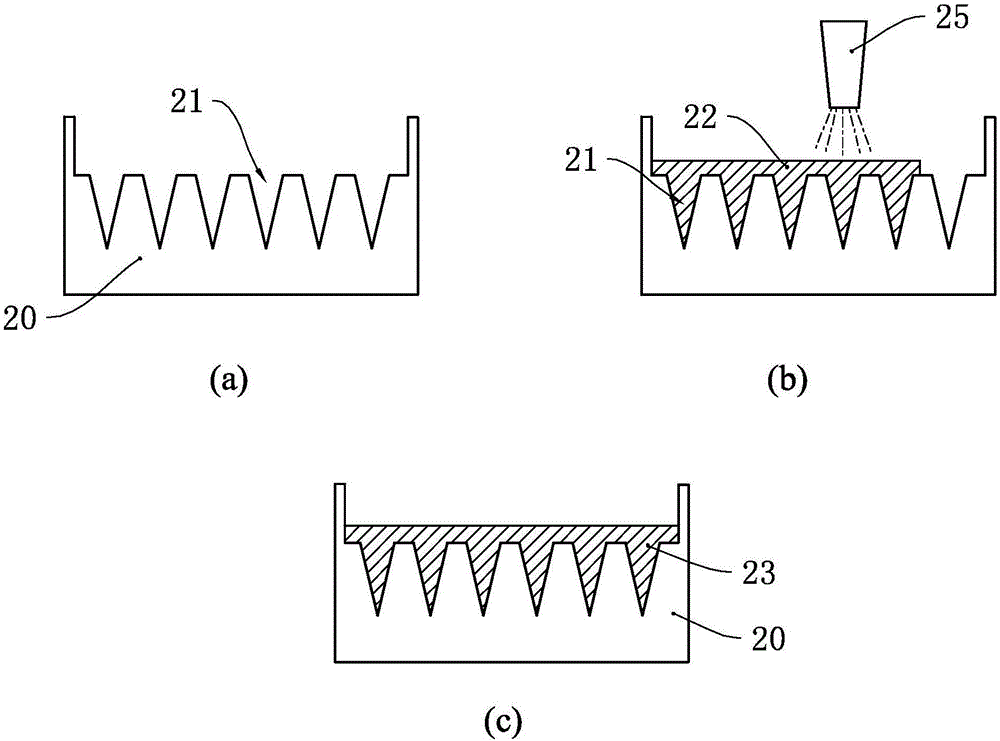
[0049] see image 3 , using this embodiment to manufacture microneedles, firstly manufacture a microneedle mold 30, and the microneedle mold 30 has a plurality of microporous cavities 31 therein. After manufacturing the microneedle mold 30, at first spray water or the active drug solution into the microneedle mold 30, that is, use the high-pressure atomization nozzle 35 of the high-pressure atomization device to spray water or the active drug solution to the microneedle mold 30, as in this embodiment The high-pressure atomizing nozzle 35 sprays water 32 to the microneedle mould. Since the viscosity of water is very low and the surface tension is very small, it can easily flow into the microporous cavity 31 .
[0050] Then, the polymer solution 33 is sprayed to the microneedle mold 30 by using a high-pressure atomizing device. Since the microporous cavity 31 has been filled with water, the polymer solution 33 is actually sprayed on the water 32 . Moreover, since the polymer s...
no. 3 example
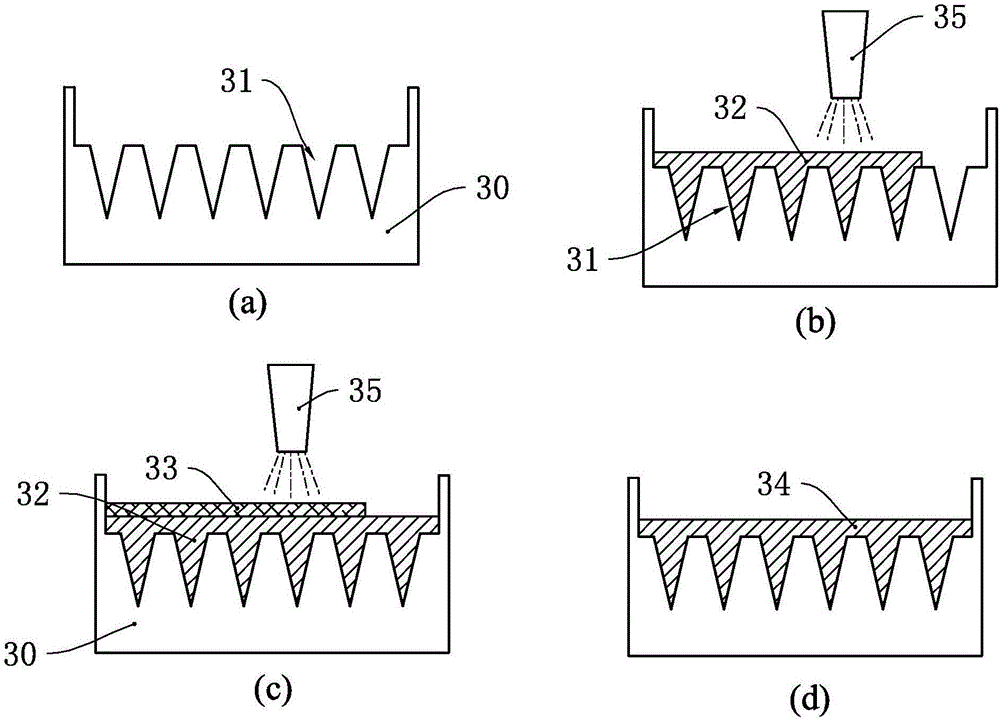
[0052] Such as Figure 4 As shown, a microneedle mold 40 is manufactured first, and a plurality of micropore cavities 41 are arranged in the microneedle mold 40 . Then, the surfactant 42 is sprayed into the microporous cavity 41 using a high-pressure atomizing device. Since the viscosity of the surfactant 42 used in this embodiment is low, and the amount injected into the microneedle mold 40 is less, the surfactant 42 only adheres to the inner wall of the micropore cavity 41 and does not fill the microneedle mold 40. Each microporous cavity 41 is filled, thereby forming a filling space 47 in the surfactant 42 .
[0053] Then, use the high-pressure atomizing nozzle 45 of the high-pressure atomizing equipment to spray the atomized polymer solution 43 on the microneedle mold 40, because the surfactant 42 has hydrophilicity, and the polymer solution 43 and the surfactant It has mutual solubility, so the polymer solution 43 can be evenly mixed on the surfactant 42, and each micro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com