Donepezil hydrochloride pharmaceutical composition and preparation method thereof
The technology of donepezil hydrochloride and adhesive is applied in the field of donepezil hydrochloride pharmaceutical composition and preparation thereof, which can solve the problems of high packaging and storage costs, and achieve the effects of improving in vitro dissolution rate, prolonging storage period and reducing drug storage conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
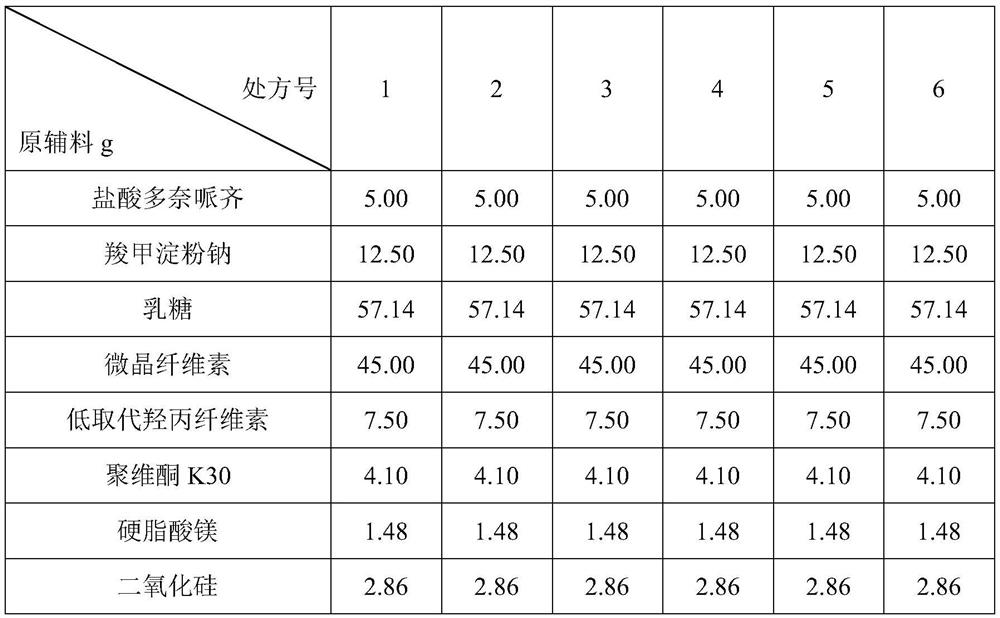
Embodiment 1
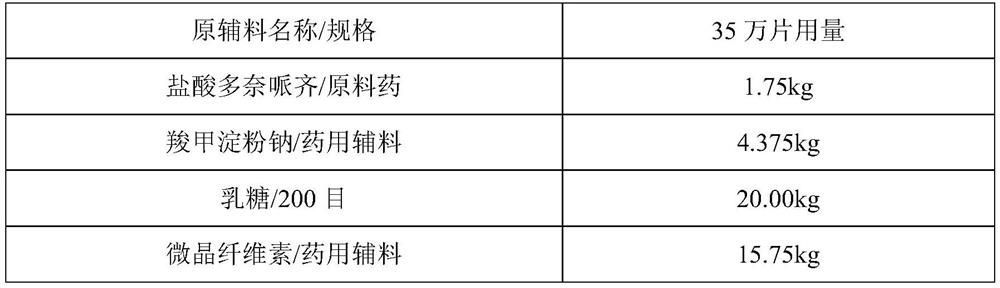
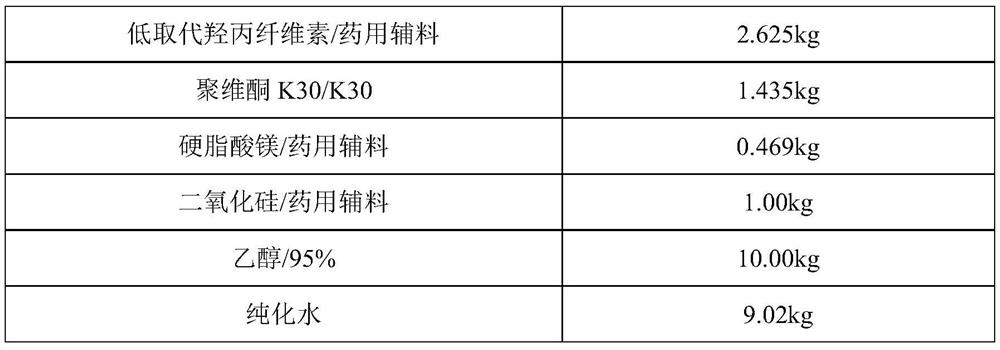
[0031] prescription:
[0032]
[0033] The particle size of the donepezil hydrochloride raw material used in different prescriptions (Malvern 2000 laser particle size analyzer, measured by dry method) is as follows:
[0034]
[0035] Among them, the raw materials of donepezil hydrochloride used in prescriptions 1 and 2 have not been micronized, and the raw materials of donepezil hydrochloride used in prescriptions 3-6 have been micronized.
[0036] Coating Solution Prescription:
[0037] Raw material name / specification Dosage Film coating premix (gastric solution type) / Y-1-7000 3.00g ethanol 34.5g purified water 12.3g
[0038] Preparation Process:
[0039] (1) Premixing: add low-substituted hydroxypropyl cellulose, carboxymethyl starch sodium, donepezil hydrochloride, lactose, and microcrystalline cellulose in the wet granulator, and initially mix evenly;
[0040] (2) Preparation of the adhesive solution: add the prescribed amount of p...
Embodiment 2
[0051] prescription:
[0052]
[0053] Carry out tabletting and coating according to the preparation technology of embodiment 1, and carry out dissolution measurement according to the assay method of embodiment 1, assay result is as follows:
[0054] Prescription 7 Prescription 8 Prescription 9 Prescription 10 Prescription 11 Prescription 12 10min 62.22 62.27 62.45 62.51 62.23 62.34 15min 100.87 100.45 100.34 100.56 100.51 100.46 30min 101.31 100.92 101.21 101.15 100.88 100.88
Embodiment 3
[0056] prescription:
[0057]
[0058]Carry out tabletting and coating according to the preparation technology of embodiment 1, and carry out dissolution measurement according to the assay method of embodiment 1, assay result is as follows:
[0059] Prescription 13 Prescription 14 Prescription 15 Prescription 16 Prescription 17 Prescription 18 10min 63.23 62.37 63.44 63.41 63.24 63.34 15min 100.83 100.43 100.32 100.42 100.34 100.43 30min 101.32 100.93 101.24 101.15 100.86 100.87
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com