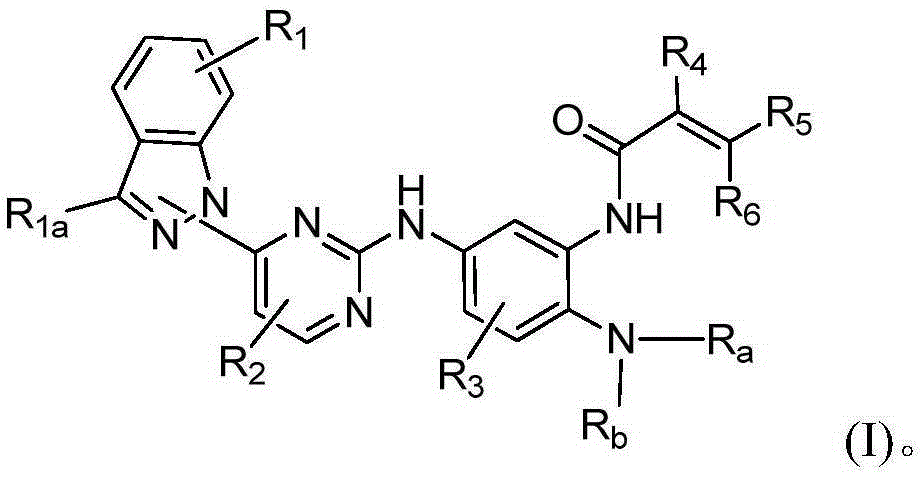
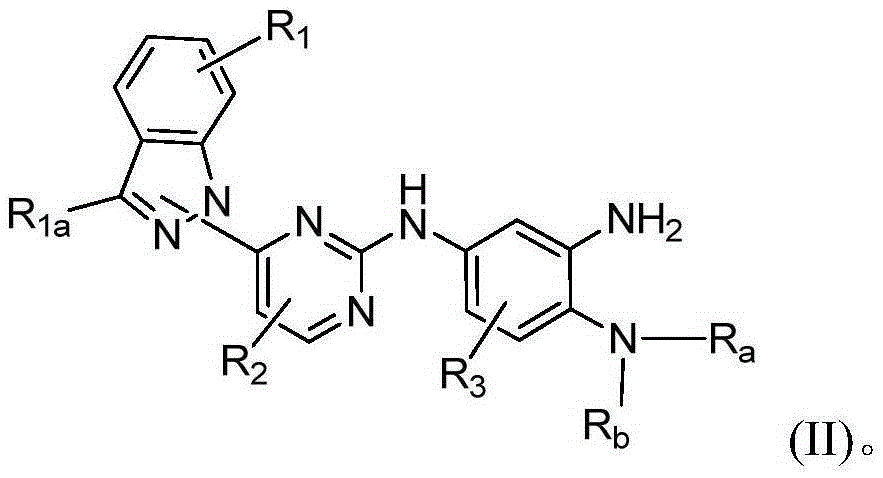
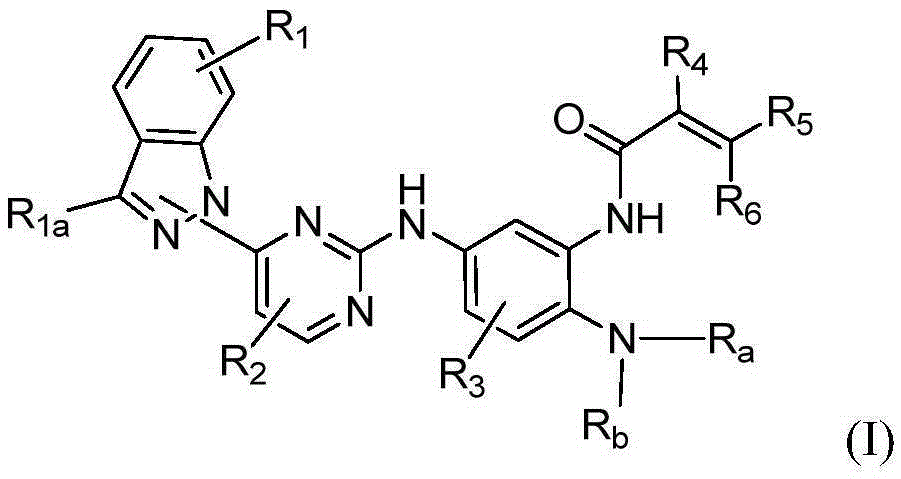
Indazole-substituted epidermal growth factor receptor (EGFR) inhibitor and application thereof
A technology of isomers and solvates, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve the lack of selectivity, affect patient compliance, and change the ATP affinity of the EGFR kinase region And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0144] Example 1A N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(1H-indazol-1-yl)pyrimidine Synthesis of -2-amino)phenyl)acrylamide
[0145]
[0146] Step a 2-Chloro-4-(1H-indazol-1-yl)pyrimidine
[0147]
[0148] In a 250mL reaction flask, add 2,4-dichloropyrimidine (14.9g, 100mmol), after 50mL of DMF dissolves, add cesium carbonate (50g, 152mmol), add dropwise 30mL of indazole (11.8g , 100mmol) DMF solution, after the dropwise addition, react at room temperature for 1h, after the reaction, pour into ice water, filter with suction, wash the filter cake with 50mL water, dry and purify by column chromatography to obtain the title compound.
[0149] ESI-Ms m / z: 231.1 [M+H].
[0150] Step b Synthesis of N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1H-indazol-1-yl)pyrimidin-2-amine
[0151]
[0152] In a 250mL reaction flask, add 2-chloro-4-(1H-indazol-1-yl)pyrimidine (16.3g, 70mmol) and 4-fluoro-2-methoxy-5-nitroaniline obtained in step a (13.0g, 70mmol) and...
Embodiment 1B
[0167] Example 1B N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(1H-indazol-2-yl)pyrimidine Synthesis of -2-amino)phenyl)acrylamide
[0168]
[0169] Synthesis of step a 2-chloro-4-(1H-indazol-2-yl)pyrimidine
[0170]
[0171] In a 250mL reaction flask, add 2,4-dichloropyrimidine (14.9g, 100mmol), after 50mL of DMF dissolves, add cesium carbonate (50g, 152mmol), add dropwise 30mL of indazole (11.8g , 100mmol) DMF solution, after the dropwise addition, react at room temperature for 1h, after the reaction, pour into ice water, filter with suction, wash the filter cake with 50mL water, dry and purify by column chromatography to obtain the title compounds respectively.
[0172] ESI-Ms m / z: 231.1 [M+H].
[0173] Step b Synthesis of N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1H-indazol-2-yl)pyrimidin-2-amine
[0174]
[0175] In a 250mL reaction flask, add 2-chloro-4-(1H-indazol-2-yl)pyrimidine (16.3g, 70mmol) and 4-fluoro-2-methoxy-5-nitroaniline obtained in ...
Embodiment 2A
[0190] Example 2A N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(5-chloro-4-(1H-indazole-1 Synthesis of -yl)pyrimidine-2-amino)phenyl)acrylamide
[0191]
[0192] Starting from 4-fluoro-2-methoxy-5-nitroaniline, 2,4,5-trichloropyrimidine, indazole, N,N,N'-trimethylethylenediamine and allyl chloride , The title compound was prepared according to the method of Example 1A.
[0193] 1 H NMR (300MHz, DMSO-d 6 )δ10.01(s,1H),9.06(s,1H),8.60(s,1H),8.45(s,1H),8.32(s,1H),8.00(d,1H),7.89(d,1H ),7.44-7.26(m,2H),7.00(s,1H),6.53(d,1H),6.24(d,1H),5.79(d,1H),3.80(s,3H),3.04-2.91( m,2H), 2.65(s,3H), 2.49-2.37(m,2H), 2.20(s,6H).
[0194] ESI-Ms m / z: 521.2 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com