Fused protein as well as preparation method and application thereof
A fusion protein and protein technology, applied in the biological field, can solve the problems of late or relapsed patients who cannot be effective, less than 50%, and fail to improve the prognosis of tumor patients, so as to achieve the effect of improving expression yield and accuracy, and improving expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
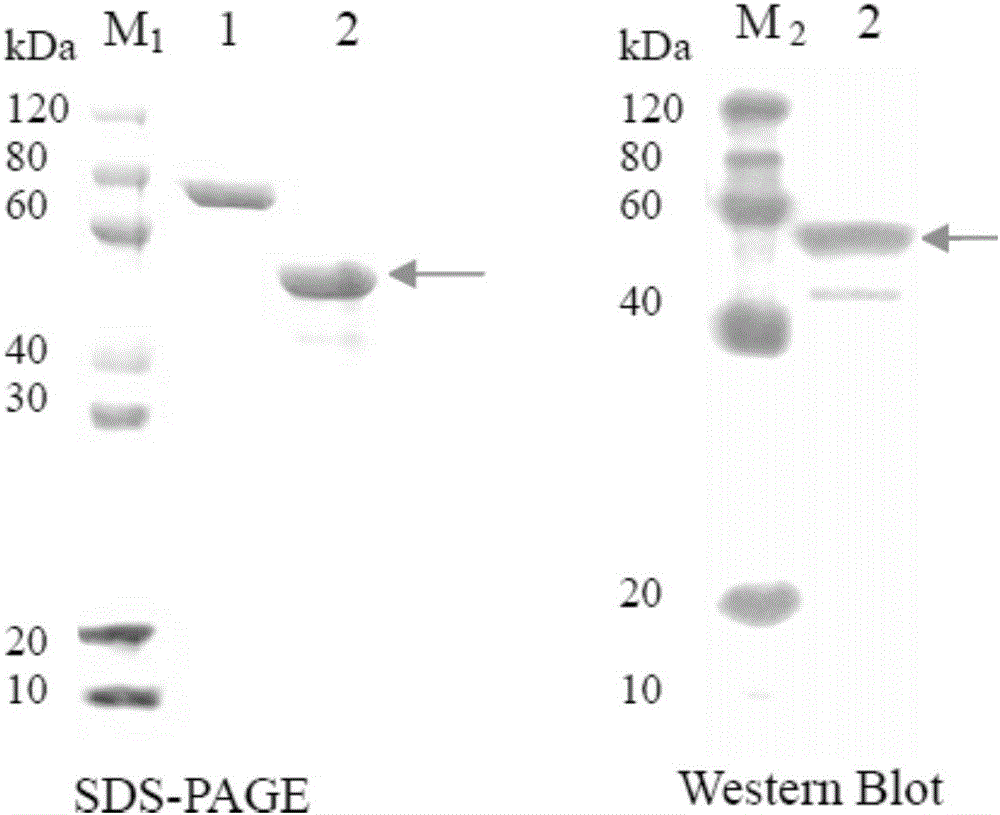
[0046] Example 1 constructs and expresses a purified fusion protein
[0047] 1. Codon optimization and gene synthesis for CLIC1 and HSP27.
[0048] The designed target nucleotide sequence is shown in SEQ ID NO.2, which can encode the amino acid sequence shown in SEQ ID NO.1.
[0049] 2. Entrust Nantong University School of Medicine to use the method of whole gene synthesis to obtain the gene fragment shown in SEQ ID NO.2, named CLIC1-HSP27 gene.
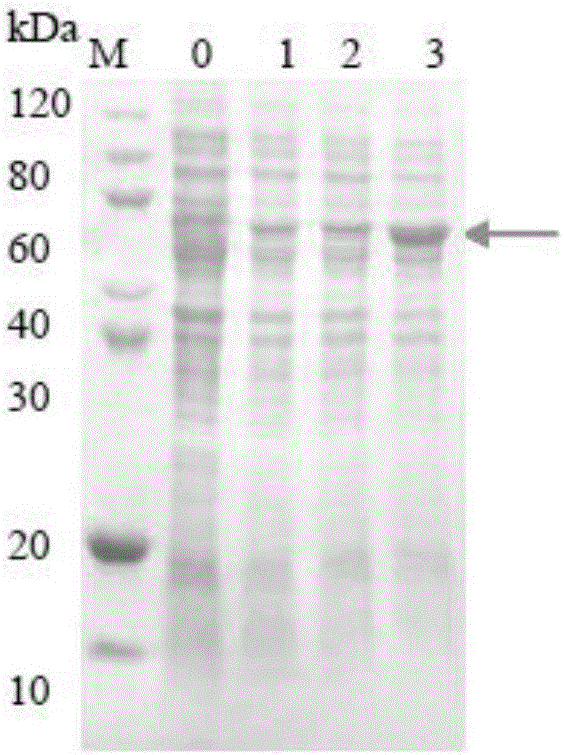
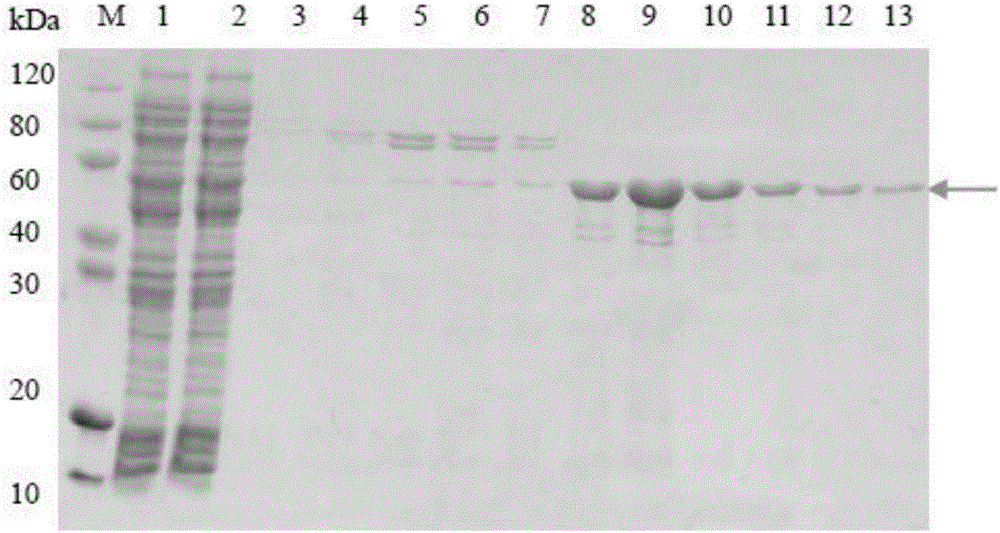
[0050] 3. Using restriction endonucleases Nde I and Hind III to digest the CLIC1-HSP27 gene and the expression vector pET30a respectively, insert the CLIC1-HSP27 gene into the expression vector pET30a to obtain recombinant plasmids, named pET30aCLIC1-HSP27, pET30a CLIC1- The plasmid map of HSP27 is as follows Figure 5 shown. In plasmid construction, the ratio of gene to vector and some related conditions are as follows (20ul reaction system):
[0051] COMPONENT 20 μl REACTION 10X T4 DNA Ligase Buffer 2ul V...
Embodiment 2
[0067] Peripheral blood mononuclear cells were obtained through the standard procedure of leukocyte separation, and co-cultured with the recombinant protein CLIC1-HSP27 in vitro to obtain antigen-presenting cells (APCs) and killer lymphocytes activated by the recombinant protein CLIC1-HSP27, and the antigen-presenting cells will be The PAP protein is digested into a polypeptide and presented on its surface, which can be recognized by T cells of the immune system. After recognizing the antigen, the T cells can find and kill cancer cells expressing the CLIC1 antigen in vivo.
Embodiment 3
[0069] The TGF-β content in the supernatant of T lymphocytes activated by CLIC1-HSP27-loaded dendritic cells was detected by Elisa method (TGF-β is a cytokine secreted when T cells are activated), and the detection results were as follows: Figure 6 As shown, blood sample A, blood sample B and blood sample C come from peripheral blood of different people respectively. From Figure 6 It can be seen that, compared with the control group (blank control group) between CLIC1-HSP27, the content of TGF-β in the CLIC1-HSP27 stimulation group was significantly increased (pg / ml).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com