Mn (IV)-activated red hexafluoride light-emitting material and preparation method
A hexafluoride, red luminescence technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of high color temperature and low color rendering index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 0.202 g and HfO 2 Dissolve in 5ml hydrofluoric acid (40wt%), stir at room temperature for 60 minutes until the dissolution is complete, add 0.062g potassium hexafluoromanganate to this solution for 30 minutes; then add 0.304g cesium fluoride solid and continue to stir for 50 minutes. The obtained precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum drying oven for 24 hours. The orange-red powder obtained was the final product Cs 2 HfF 6 :Mn 4+ .
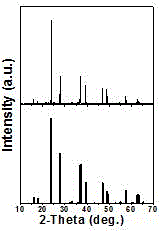
[0017] The XRD diffraction pattern of this phosphor is attached figure 1 As shown, the diffraction peaks of the sample are compared with the standard card JCPDS 74-0175 (Cs 2 HfF 6 ) Are completely consistent, and no diffraction peaks of any impurity phase are observed, which indicates that the samples we synthesized have a single crystal phase.
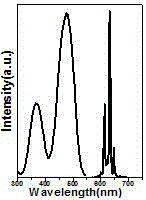
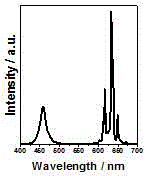
[0018] Attached figure 2 Shown are the room temperature excitation spectrum (monitoring wavelength is 631 nm) and emission...
Embodiment 2
[0022] Weigh 0.197 g and HfO 2 Dissolve in 10 ml of hydrofluoric acid (40wt%), stir for 30 minutes in a water bath at 50°C until the dissolution is complete, add 0.124g potassium hexafluoromanganate to this solution and react for 30 minutes; then add 0.456 g cesium fluoride solid Continue to stir for 60 min. The obtained precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum drying oven for 24 hours. The orange-red powder obtained was the final product Cs 2 HfF 6 :Mn 4+ .
Embodiment 3
[0024] Weigh 0.202 g and HfO 2 Dissolve in 10 ml of hydrofluoric acid (40wt%), stir for 10 minutes in a water bath at 80°C until the dissolution is complete, add 0.062g potassium hexafluoromanganate to this solution and react for 30 minutes; then add 0.608 g cesium fluoride solid Continue to stir for 60 min. The obtained precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum drying oven for 24 hours. The orange-red powder obtained was the final product Cs 2 HfF 6 :Mn 4+ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com