A rapid qualitative and quantitative detection kit for oil adjuvant vaccine and its detection method
A quantitative detection and kit technology, applied in the direction of biological testing, preparation of test samples, material inspection products, etc., can solve the problems of inability to directly detect, demulsification, lack of unified and accurate inspection of foot-and-mouth disease vaccine, etc., and achieve rapid Effective detection means, accurate detection results, and the effect of improving the efficiency of demulsification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
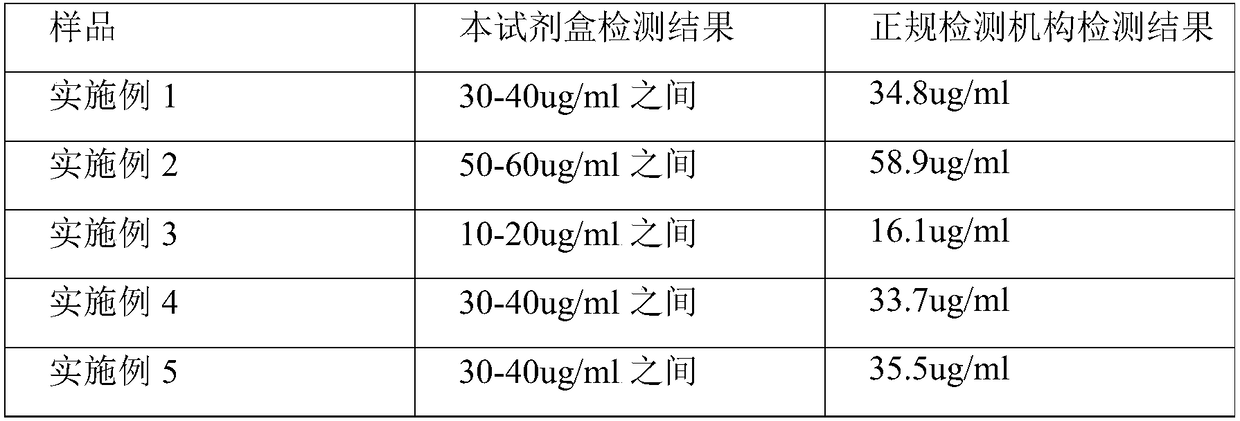
[0027] This embodiment provides a rapid qualitative and quantitative detection kit for oil adjuvant vaccines, including the following components: ethanol (containing 0.33% hydrochloric acid by volume), syringe, rapid detection test paper, and color comparison card.
[0028] The rapid detection test paper is impregnated with a mixture of G-250, ethanol and phosphoric acid, wherein the final concentration of G-250 is 0.1mg / ml, the final concentration of ethanol is 5%, and the final concentration of phosphoric acid is 8.5%.
[0029] The color card is made after using the above-mentioned test paper to develop the color of standard BSA solutions of different concentrations. Standard BSA solution concentrations are: 10ug / ml, 20ug / ml, 30ug / ml, 40ug / ml, 50ug / ml, 60ug / ml, 70ug / ml, 80ug / ml, 90ug / ml, 100ug / ml.
[0030] The detection method using the above-mentioned rapid detection kit comprises the following steps:
[0031] 1. Take out 0.7ml of vaccine sample 1 with a syringe, and then ...
Embodiment 2
[0036] This embodiment provides a rapid qualitative and quantitative detection kit for oil adjuvant vaccines, including the following components: ethanol (containing 0.33% hydrochloric acid by volume), syringe, rapid detection test paper, and color comparison card.
[0037] The rapid detection test paper is impregnated with a mixture of G-250, ethanol and phosphoric acid, wherein the final concentration of G-250 is 0.1mg / ml, the final concentration of ethanol is 5%, and the final concentration of phosphoric acid is 8.5%.
[0038] The color card is made after using the above-mentioned test paper to develop the color of standard BSA solutions of different concentrations. Standard BSA solution concentrations are: 10ug / ml, 20ug / ml, 30ug / ml, 40ug / ml, 50ug / ml, 60ug / ml, 70ug / ml, 80ug / ml, 90ug / ml, 100ug / ml.
[0039] The detection method using the above-mentioned rapid detection kit comprises the following steps:
[0040] 1. Take out 0.9ml of vaccine sample 2 with a syringe, then take...
Embodiment 3
[0045] This embodiment provides a rapid qualitative and quantitative detection kit for oil adjuvant vaccines, including the following components: acetonitrile (containing 0.05% trifluoroacetic acid), syringe, rapid detection test paper, and color comparison card.
[0046] The rapid detection test paper is impregnated with a mixture of G-250, ethanol and phosphoric acid, wherein the final concentration of G-250 is 0.1mg / ml, the final concentration of ethanol is 5%, and the final concentration of phosphoric acid is 8.5%.
[0047] The color card is made after using the above-mentioned test paper to develop the color of standard BSA solutions of different concentrations. Standard BSA solution concentrations are: 10ug / ml, 20ug / ml, 30ug / ml, 40ug / ml, 50ug / ml, 60ug / ml, 70ug / ml, 80ug / ml, 90ug / ml, 100ug / ml.
[0048] The detection method using the above-mentioned rapid detection kit comprises the following steps:
[0049] 1. Take out 0.5ml of vaccine sample 3 with a syringe, then take o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com