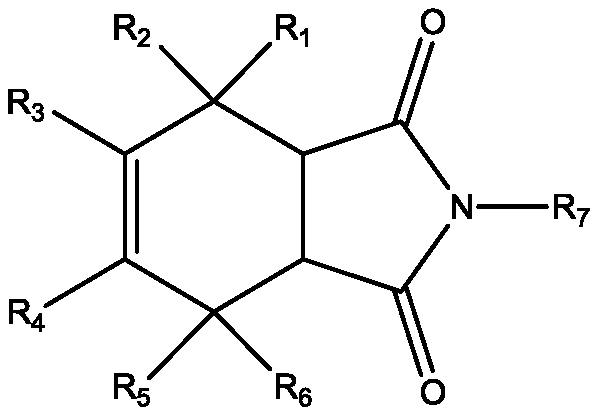
A new application of isoindole-1,3-dione compound
The technology of a compound, isoindole, is applied in the field of isoindole-1 to achieve the effect of inhibiting the activity of calcium-dependent protein kinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
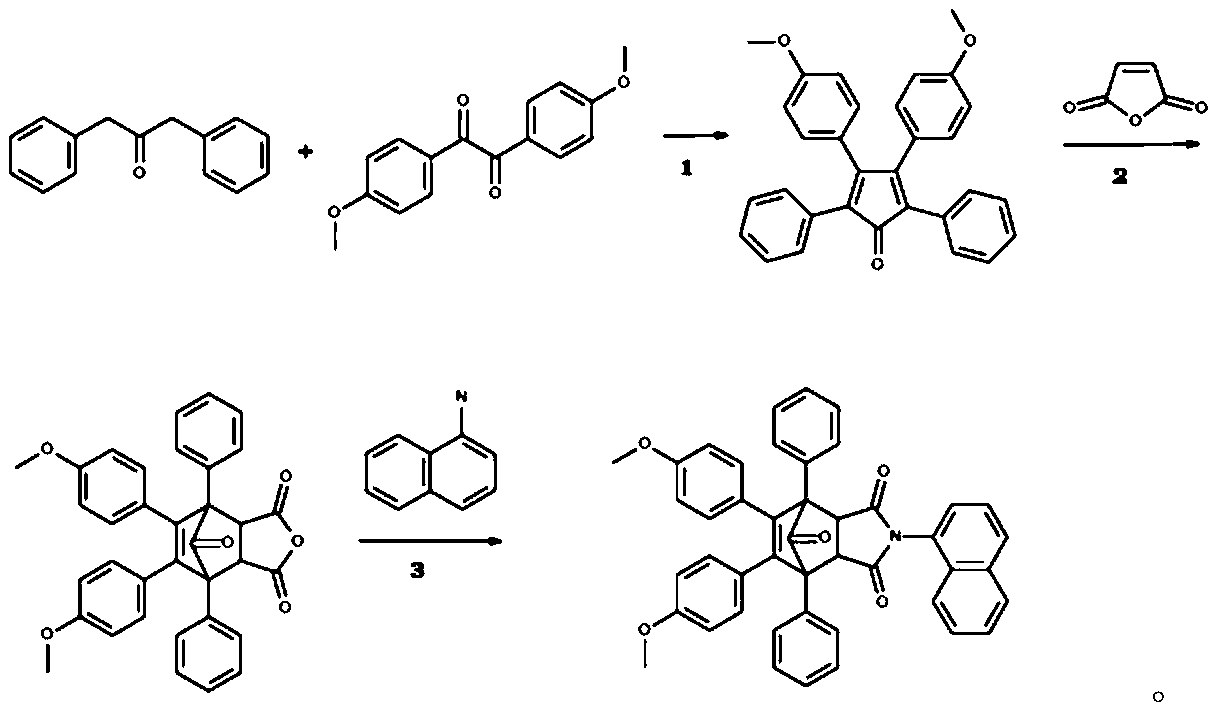
[0019] Example 1: Preparation of isoindole-1,3-dione compound I.
[0020] 8,9-bis(4-methoxyphenyl)-4-(1-naphthyl)-1,7-diphenyl-4-azatricyclo[5.2.1.0~2,6~]decane- The synthetic route of 8-ene-3,5,10-trione is as follows:
[0021]
[0022] The first step refers to [1], the second step refers to [2], and the third step refers to [3,4].
[0023] 1. Cesari, C., et al., Microwave-Assisted Synthesis of Functionalized Shvo-Type Complexes. Organometallics, 2014.33(11): p.2814–2819.
[0024] 2. e-EROS Encyclopedia of Reagents for Organic Synthesis, I. John Wiley and Sons, Editor. 2001.
[0025] 3. Lai, Y.-H., et al., Structural studies on phenanthro[9,10-e]pyrene and its 9,10-dihydro-derivative: dependence of chemical shift on degree of planarity of and buttressing effect in the polycyclic aromatic compounds .Journal of the Chemical Society,Perkin Transactions 2,1992(8):p.1315-1319.
[0026] 4. Carroll, W.R., et al., A Molecular Balance for Measuring Aliphatic CH-πInteractions. O...
Embodiment 2
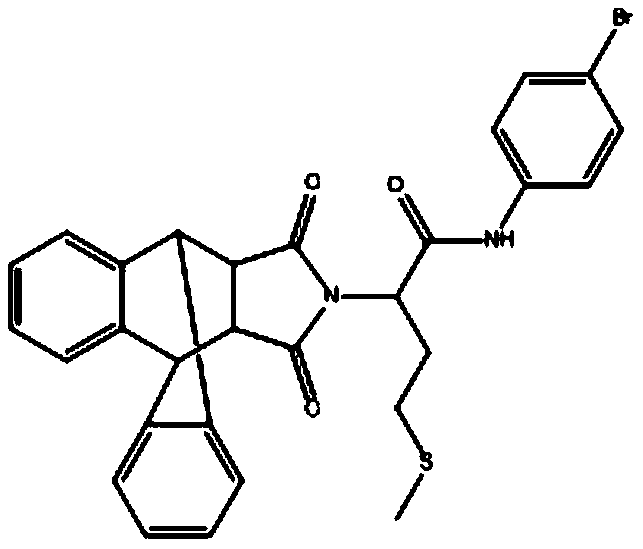
[0027] Example 2: Determination of the inhibitory activity of the isoindole-1,3-dione compounds I, II and III prepared in the present invention against CDPK1.
[0028] Compound II is N-(4-bromophenyl)-2-(16,18-dioxo-17-azapentacyclo[6.6.5.0~2,7~.0~9,14~.0~15 ,19~]nonadecane-2,4,6,9,11,13-hexene-17-substituted)-4-(methylthio)butanamide, the structural formula is as follows:
[0029]
[0030] Compound III is phenyl 1,14-dimethyl-4,10-bis(2-methylphenyl)-3,5,9,11-tetraoxo-4,10-diazatetracyclo[5.5.2.0 ~2,6~.0~8,12~]tetradecane-13-ene-13-carboxylate, the structural formula is as follows:
[0031]
[0032] Both compound II and compound III were purchased from Specs Company.
[0033] 1 experimental equipment
[0034] Fluorescence spectrophotometer (Shimadzu RF-5301PC, Japan shimadzu), electric heating constant temperature water bath (Kewei), low-speed centrifuge (Feige brand), 1mL, 200uL, 100uL, 10uL pipette guns (Biohit), 1.5mL EP tube (Solaibao), 200uL PCR tube (Solaibao)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com