Recombinant human VEGF tumor polypeptide vaccine
A tumor vaccine, tumor technology, applied in the field of medical biotechnology and immunology, can solve the problem of less immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Construction of recombinant human VEGF polypeptide vaccine prokaryotic expression plasmid
[0015] A synthetic method was used to obtain the C-terminal with Bam H I and EcoR The phage Qβ coat protein gene expression sequence at the Ⅰ restriction site was cloned into the first expression unit of the vector pRSFDuet1, and the synthetic upstream was introduced Bam HI, downstream introduction Eco The human VEGF antigen peptide nucleic acid sequence of the RI restriction site is connected to the C-terminus of the Qβ coat protein gene expression sequence; the phage Qβ coat protein gene expression sequence is inserted into the second expression unit of the vector pRSFDuet1.
Embodiment 2
[0016] Example 2: Prokaryotic expression of recombinant human VEGF polypeptide vaccine
[0017] Transform the recombinant plasmid into Escherichia coli BL21 (DE3) competent cells, pick a single clone into LB medium containing kanamycin (50mg / mL), culture overnight for 12-16 hours, and then press the ratio of 1:20 Transfer to fresh LB medium containing kanamycin and continue culturing for 2-3 hours. When the OD value of the bacterial solution reaches 0.5, add IPTG (1 mmol / L) to induce expression for 3 hours, and the induction temperature is 37°C.
Embodiment 3
[0018] Embodiment 3: Purification of recombinant human VEGF polypeptide vaccine
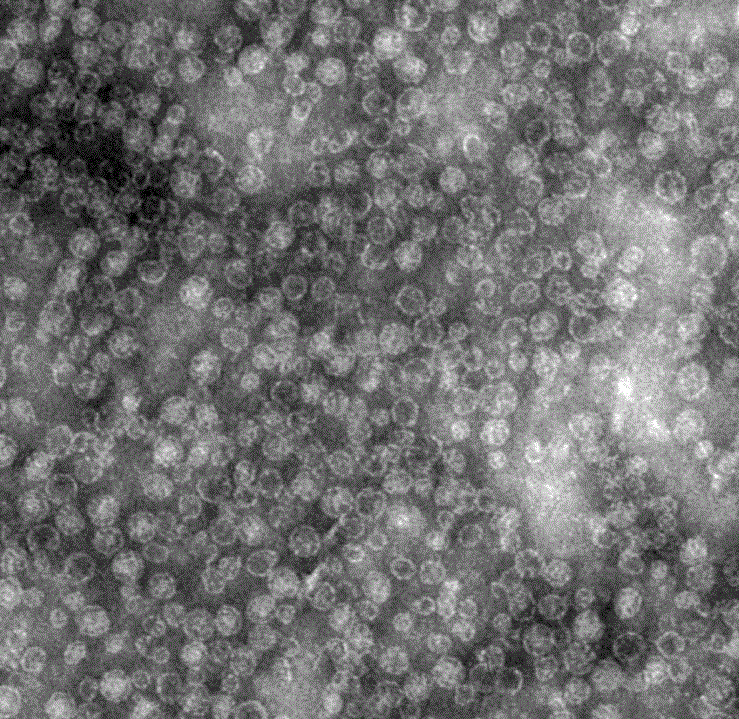
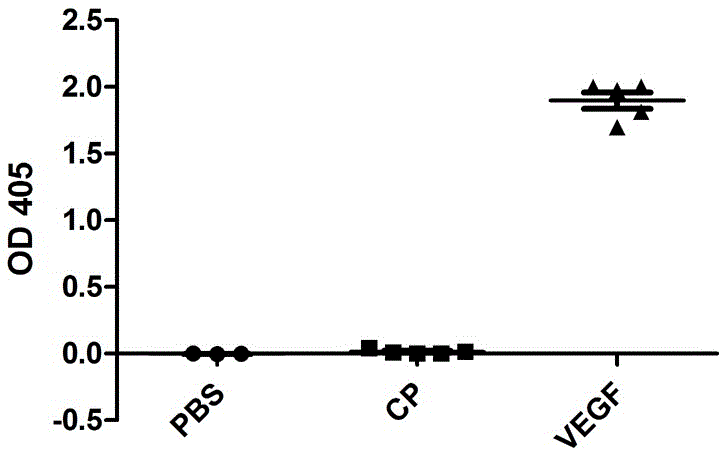
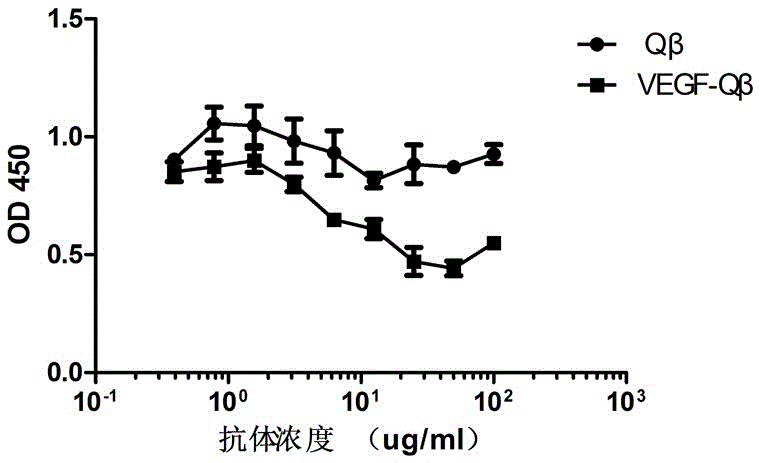
[0019] The induced bacterial solution was centrifuged at 4500 rpm for 20 min to collect the bacterial cells, and the bacterial cells were resuspended in PBS and then ultrasonically disrupted under the conditions of 15% power, working for 5 sec, stopping for 5 sec, and the total time was 10 min. The ultrasonically crushed sample was centrifuged at 12000 rpm for 10 min, the supernatant was added with ammonium sulfate to 40% saturation, mixed for 30 min at 4 °C, centrifuged at 12000 rpm for 10 min, the supernatant was discarded, the precipitate was dissolved in PBS, and the lysate was dissolved in 10 min -50% sucrose solution was subjected to density gradient centrifugation (40,000 rpm, 3 h), and the bands containing virus-like particles were taken out, and the sucrose was replaced with PBS through a G-25 desalting purification column to obtain a purified recombinant human VEGF polypeptide vaccine ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com