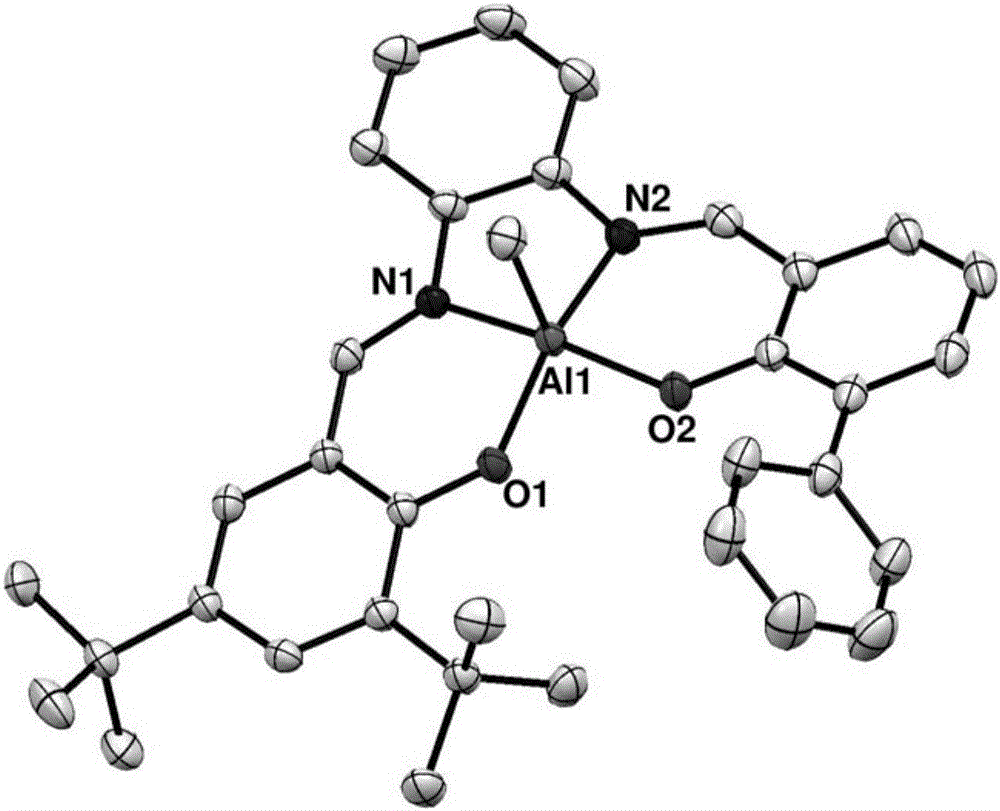
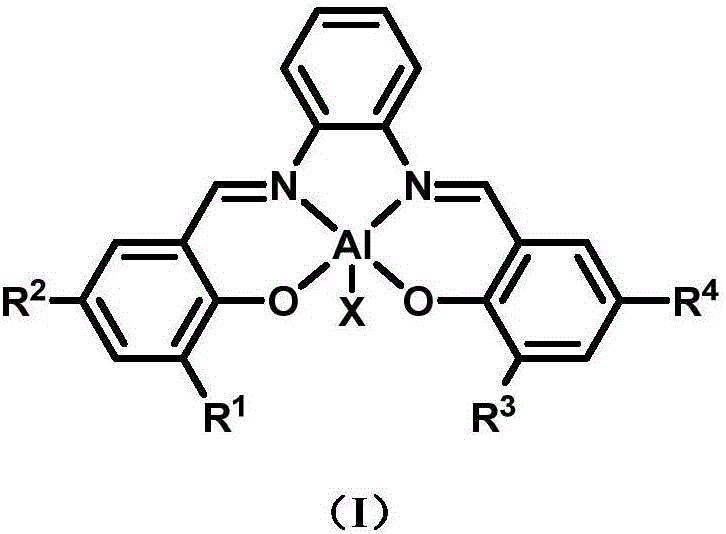
Preparation method and application of asymmetric N,N'-bis(salicylaldehyde)o-phenylenediamine aluminum compound
A technology of o-phenylenediamine aluminum and o-phenylenediamine alkylaluminum, which is applied in the field of preparation of organic aluminum compounds, can solve problems such as cumbersome synthesis steps, and achieve the effects of convenient preparation, low cost and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, intermediate monosalicylaldimine (3,5- t Bu-2-(OH)C 6 h 2 CH=N-C 6 h 4 -NH 2 ) preparation.
[0033] Dissolve o-phenylenediamine (2.16g, 20mmol), 3,5-di-tert-butyl salicylaldehyde (2.34g, 10mmol) and catalytic amount of p-toluenesulfonic acid (20mg) in ethanol, at room temperature The reaction was stirred for 12 hours. The reaction liquid was concentrated under reduced pressure and filtered to obtain the monosalicylaldimine intermediate (2.85 g, 8.8 mmol, 88%). 1 H NMR (CDCl 3):δ13.40(s,1H,OH),8.64(s,1H,CH=N),7.45(s,1H),7.23(s,1H),7.09(t,1H,J=7.5Hz), 7.04(d,1H,J=7.5Hz),6.83-6.75(m,2H),4.41-3.39(br,2H,NH 2 ),1.50(s,9H,CMe 3 ),1.32(s,9H,CMe 3 ).Anal.Calcd for C 21 h 28 N 2 O: C, 77.74; H, 8.70; N, 8.63. Found: C, 77.75; H, 8.62; N, 8.55.
Embodiment 2
[0034] Example 2, unsymmetrical N, N'-bis(salicylaldehyde) phenylenediamine (3,5- t Bu-1-OH-C 6 h 2 )CH=N-C 6 h 4 -N=CH(3-Ph-1-OH-C 6 h 3 ) Preparation of Ligand L.
[0035] With the intermediate monosalicylaldimine (3,5- t Bu-2-(OH)C 6 h 2 CH=N-C 6 h 4 -NH 2 ) was dissolved in ethanol, 3-phenyl salicylaldehyde (1.74g, 8.8mmol) and p-toluenesulfonic acid (20mg) of catalyst amount were added, and reacted at room temperature for 12 hours. The reaction solution was concentrated under reduced pressure and filtered to obtain the unsymmetrical N,N'-bis(salicylaldehyde)o-phenylenediamine ligand (3.76 g, 7.5 mmol, 85%). 1 H NMR (CDCl 3 ):δ13.66(s,1H,OH),13.52(s,1H,OH),8.72(s,1H,CH=N),8.64(s,1H,CH=N),7.66(d,2H, J=7.5Hz),7.49-7.43(m,2H),7.41-7.38(m,3H),7.36-7.31(m,3H),7.26-7.18(m,3H),7.00(t,1H,J= 7.8Hz), 1.45(s, 9H, CMe 3 ),1.32(s,9H,CMe 3 ). 13 C NMR (CDCl 3 ):δ164.89,164.16,158.86,158.71,142.84,142.41,140.58,137.72,137.30,134.37,132.00,130.14,129.58,128.40,128.15,12...
Embodiment 3
[0036] Embodiment 3, the preparation of aluminum compound LAlMe.
[0037] Under a nitrogen atmosphere, dissolve ligand L (1.01 g, 2 mmol) in 50 mL of toluene, and slowly add 1.1 equivalents of AlMe 3 (1.1mmol, 1.1mL, 1M toluene solution), stirred at room temperature for 16 hours. The toluene solvent was removed under reduced pressure, and n-hexane (3×10 mL) was added to wash to obtain 1.03 g of a pale yellow solid, 1.90 mmol, 95%. 1 H NMR (C 6 D. 6 ):δ8.02(s,1H,CH=N),7.98(s,1H,CH=N),7.92(d,2H,J=7.2Hz),7.76(s,1H),7.50(d,1H ,J=7.0Hz),7.41(t,2H,J=7.4Hz),7.28(t,1H,J=7.4Hz),6.99(s,1H),6.93(d,1H,J=7.6Hz), 6.90-6.84(m,2H),6.75-6.68(m,3H),1.54(s,9H,CMe 3 ),1.38(s,9H,CMe 3 ),-0.39(s,3H,AlMe). 13 C NMR (C 6 D. 6 ):δ164.67,162.35,161.86,141.80,139.55,139.11,138.93,138.38,137.71,134.74,133.69,132.03,129.79,128.75,128.31,128.17,127.41,126.55,119.62,118.17,116.82,116.30,116.20,35.21, 34.17, 31.45, 29.10, -10.08. Anal. Calcd for C 35 h 37 AlN 2 o 2 : C, 77.18; H, 6.85; N, 5.14....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com