Method used for preparing unsaturated aromatic ester from phenylacetylene via carbonylation
A phenylacetylene and unsaturated technology, which is applied in the field of polymer-supported palladium-based catalysts, can solve problems that affect industrial applications, that homogeneous catalysts are difficult to recycle, and increase production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
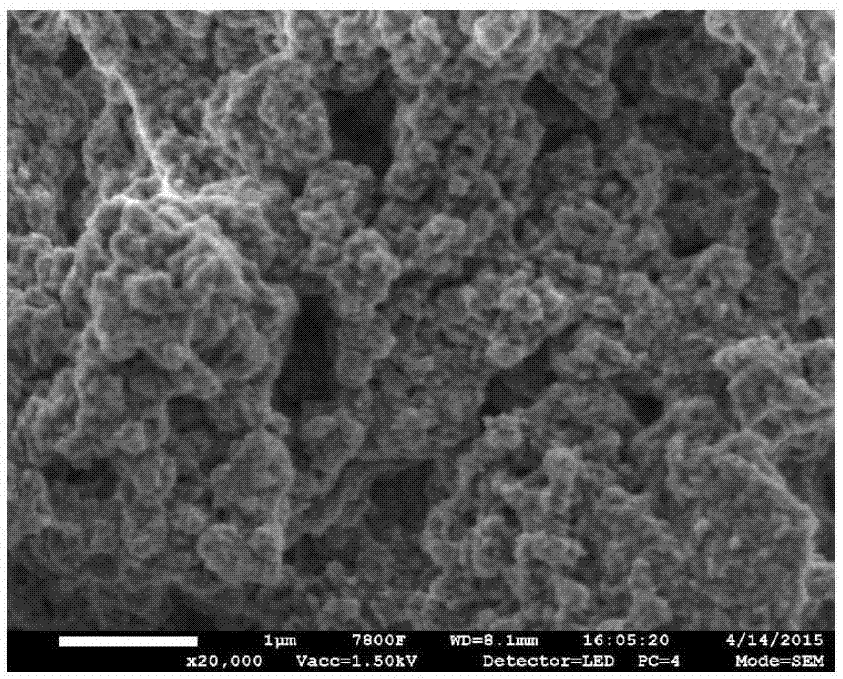
Image
Examples
Embodiment 1
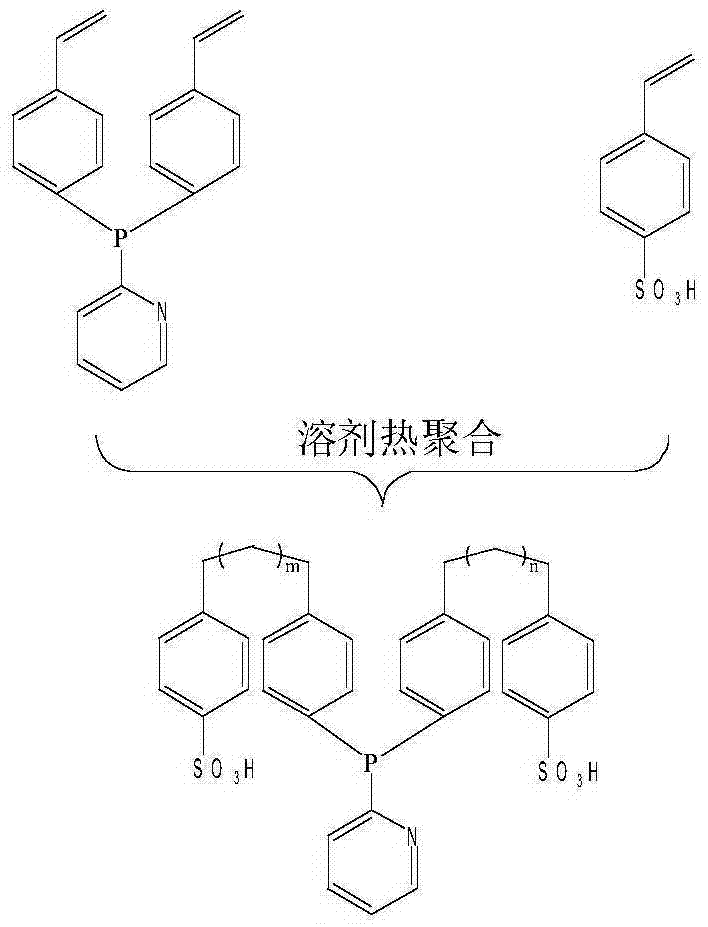
[0019] POL-2V-P,N-SO 3 H Preparation method: In a liquid nitrogen bath and a nitrogen atmosphere, add 45ml of tetrahydrofuran, 0.12mol of n-butyllithium, 0.1mol of o-bromopyridine, and 0.135mol to a 1L three-necked round-bottomed flask with a magnetic stirrer ZnCl 2 , 45ml pyridine, 90ml tetrahydrofuran, and react at room temperature for 14h. Pour out the liquid in the bottle under nitrogen protection, then add 60ml pyridine, 180ml tetrahydrofuran, and 0.12mol of PCl3 solution (dissolved in 60ml pyridine, 60ml tetrahydrofuran) under liquid nitrogen bath conditions, then react at room temperature for 26h (named reaction Liquid 1). In a liquid nitrogen bath and a nitrogen atmosphere, add 0.22mol (5.4g) of magnesium chips, 35ml of tetrahydrofuran, and 36.6g (0.2mol) of p-bromostyrene to another 1L three-necked round-bottomed flask with a magnetic stirrer (named as reaction solution 2). After the reaction solution 1 was filtered, it was added to the reaction solution 2 in a li...
Embodiment 2
[0022] Reaction temperature is 40 ℃, and other conditions are with embodiment 1.
Embodiment 3
[0024] Reaction temperature is 50 ℃, and other conditions are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com