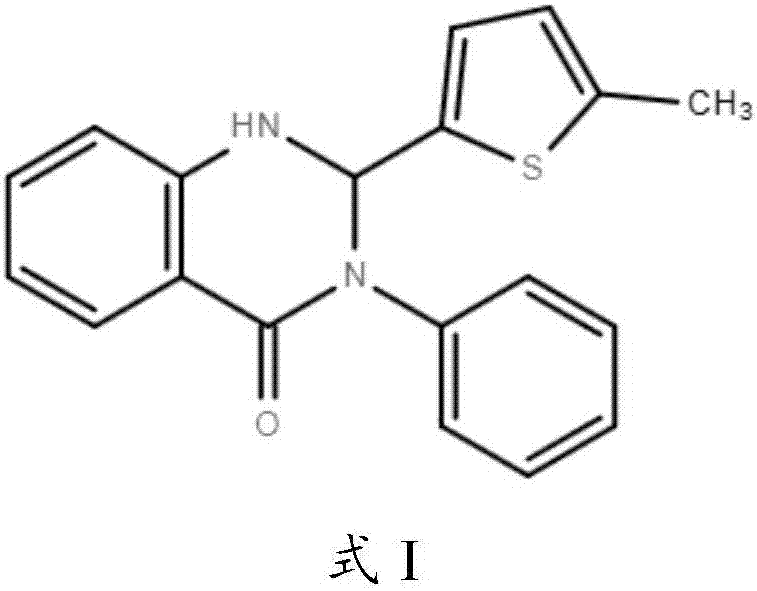
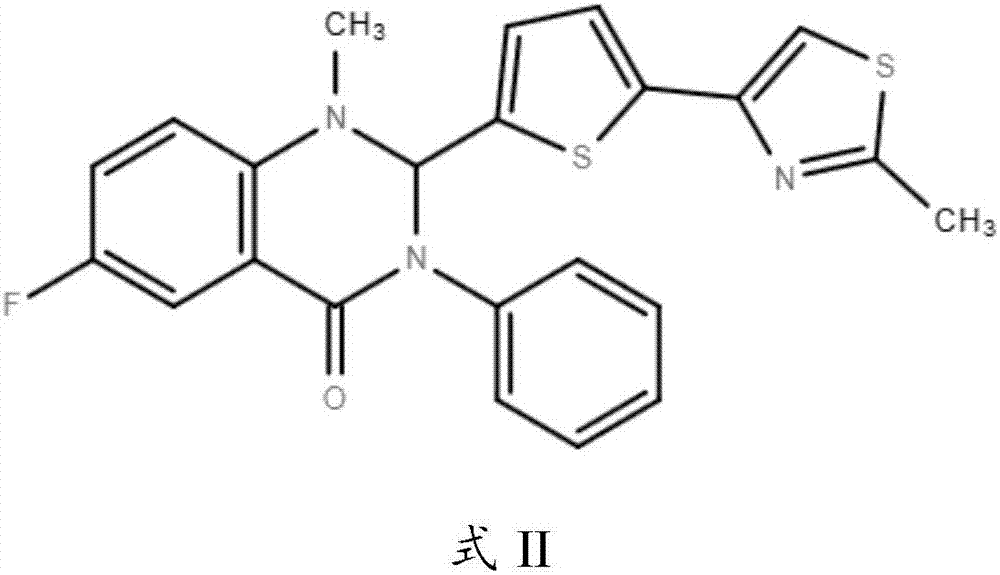
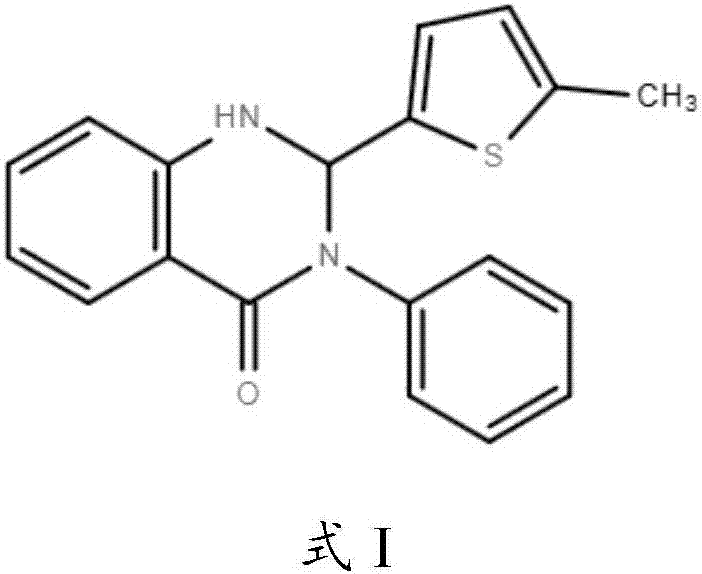
Application of Retro-2cycl and related derivatives
A retro-2.1, derivative technology, applied in the field of new drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Construction of 293S cells
[0100] The synthetic EV71 receptor human SCARB2 gene fragment (GenBank accession no: NM_005506.3, synthesized by Shanghai Jierui Company) with EcoR I & Xba I restriction sites at both ends was inserted into the lentiviral vector Lenti-XpLVX-Puro vector (purchased from Clontech), and use the transfection reagent in the lentiviral vector kit (purchased from Clontech) to transfect human embryonic kidney cells (HEK 293 cells, purchased from ATCC, accession number #CRL-1573) according to the instructions to obtain stable transfection human SCARB2 HEK 293 cells, referred to as 293S cells.
Embodiment 2
[0101] Example 2: Retro-2 cycl and Retro-2.1 cytotoxicity and inhibition of cytopathic effects caused by enterovirus 71
[0102] Take 293S cells in the logarithmic growth phase, and use 3×10 4 The density of cells / well was inoculated in 96-well culture plate and cultured for 24 hours. 293S cells at 37°C and 5% CO 2 The culture medium used was DMEM cell culture medium containing 1.25 μg / ml puromycin (purchased from Clontech) supplemented with 10% fetal bovine serum (FBS) (abbreviated as 10% FBS-DMEM, both FBS and DMEM were purchased from Sigma).
[0103] In detection of Retro-2 cycl When the cytotoxicity of Retro-2.1 and Retro-2.1 was detected, the original cell culture medium was sucked off, and 100 μl of Retro-2 containing different concentrations were added to each well. cycl and Retro-2.1 supplemented with 2% fetal bovine serum DMEM cell culture fluid (abbreviated as 2% FBS-DMEM), so that Retro-2 cycl And the final concentration of Retro-2.1 is 500μM, 250μM, 125μM, 62...
Embodiment 3
[0124] Example 3: Retro-2 cycl Inhibition of viral plaque effect caused by enterovirus 71 and Retro-2.1
[0125] Take 293S cells in the logarithmic growth phase, and use 5×10 5 The density of cells / well was inoculated in 12-well culture plate, and cultivated for 24 hours. 293S cells at 37°C and 5% CO 2 Next, the culture medium used was 10% FBS-DMEM containing 1.25 μg / ml puromycin. Aspirate the original cell culture solution from each well, and before adding 500 μl EV71 to infect (the infection volume is 100PFU / well), add 500 μl of Retro-2 containing different concentrations to the cells in each well cycl and Retro-2.1 in 2% FBS-DMEM cell culture medium to make Retro-2 cycl The final concentrations of Retro-2.1 and Retro-2.1 were 25 μM, 12.5 μM, 6.25 μM, 3.13 μM, 1.56 μM and 6.25 μM, 1.56 μM, 0.39 μM, 0.098 μM, 0.024 μM, and each concentration was repeated for 5 hours; Add EV71 to infect and maintain the presence of the drug, continue to culture the cells for 1 hour, suck ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com