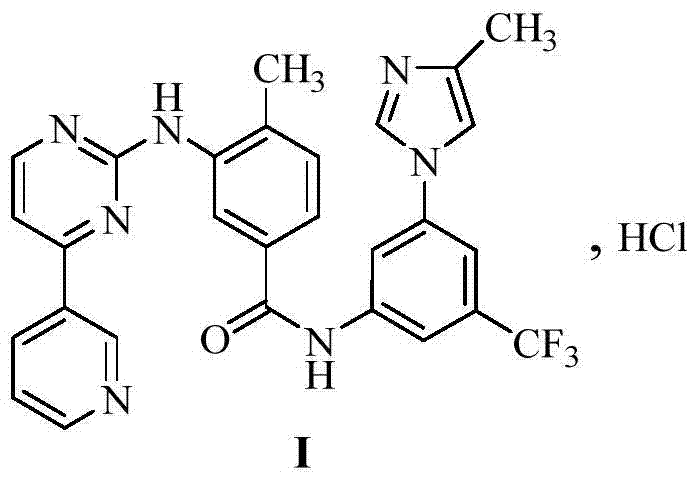
Preparation method of erlotinib hydrochloride impurities
A technology of nilotinib hydrochloride and impurities is applied in the field of 4-methyl, an important impurity in the preparation process, to achieve the effects of reducing side reactions, convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
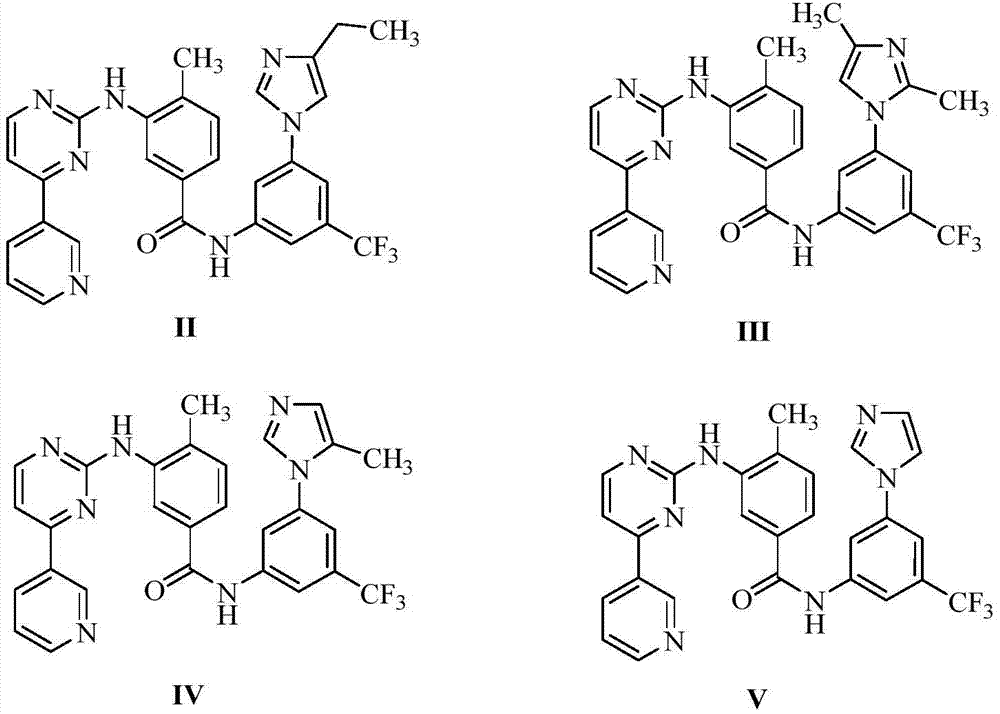
[0026] Embodiment 1: the preparation of formula II compound
[0027]
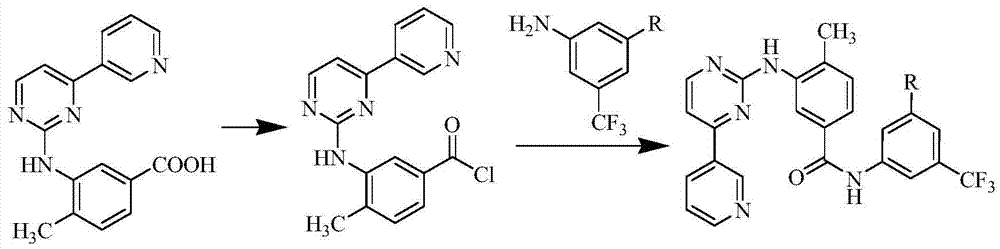
[0028] The compound of formula VI (3.5 g), toluene (35 ml), and thionyl chloride (14 ml) were added into the reaction flask, and the temperature was raised to 80° C. for 5 h. The reaction solution was spin-dried, 3-(4-ethyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline (2.0 g) and acetonitrile (70 ml) were added, and the temperature was raised to 80° C. for 7 h. Concentrate the reaction solution to obtain a black oil, which is separated and purified with a silica gel column. The eluent is dichloromethane:methanol=30:1. The product is collected and concentrated to obtain 2.9g of the compound of formula II. The molar yield is 69%, HPLC purity 98.40%. ESI-MS(m / z):543.9[M+H] + ; 1 H NMR (400MHz, DMSO-d 6 )δ: 10.62(s, 1H, CONH), 9.30(s, 1H, ArH), 9.18(s, 1H, NH), 8.70(s, 1H, ArH), 8.56(d, J=4.8Hz, 1H, ArH), 8.46(d, J=8.0Hz, 1H, ArH), 8.46(d, J=8.0Hz, 1H, ArH), 8.34(d, J=1.6Hz, 1H, ArH), 8.31(s, 1H , ArH)...
Embodiment 2
[0029] Embodiment 2: the preparation of formula III compound
[0030]
[0031]The compound of formula VI (3.5 g), tetrahydrofuran (25 ml), and oxalyl chloride (15 ml) were added into the reaction flask, and the temperature was raised to 60° C. for 6 h. Spin the reaction solution to dryness, add 3-(2,4-dimethyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline (2.0g), toluene (100ml), and heat up to 70°C Reaction 6h. Concentrate the reaction solution to obtain a black solid, which is separated and purified with a silica gel column, the eluent is dichloromethane:methanol=50:1, the product is collected, and after concentration, 2.5g of the compound of formula III is obtained, with a molar yield of 59% , HPLC purity 99.03%. ESI-MS(m / z):543.9[M+H] + ; 1 H NMR (400MHz, CDCl 3 ):9.32(d,J=2.0Hz,1H,ArH),8.85(d,J=1.6Hz,1H,ArH),8.69(dd,J=4.8,1.6Hz,1H,ArH),8.66(s, 1H, CONH), 8.56(d, J=5.6Hz, 1H, ArH), 8.39(dt, J=8.0, 2.0, 1.6Hz, 1H, ArH), 8.10(d, J=1.6Hz, 1H, ArH) ,7.91(s,1H,ArH),7.6...
Embodiment 3
[0032] Embodiment 3: the preparation of formula IV compound
[0033]
[0034] The compound of formula VI (0.4g), acetonitrile (4ml) and thionyl chloride (1.8ml) were added into the reaction flask, and the temperature was raised to 80°C for 5h. The reaction solution was spin-dried, acetonitrile (10ml), 3-(5-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline (0.2g) were added, and the temperature was raised to 60°C for 4h. Concentrate the reaction solution to obtain a yellow solid, which is separated and purified by a silica gel column, the eluent is dichloromethane:methanol=40:1, the product is collected, and after concentration, 0.27g of the compound of formula IV is obtained, with a molar yield of 61% , HPLC purity 99.49%. ESI-MS(m / z):529.9[M+H] + ; 1 H NMR (400MHz, CDCl 3 )δ: 9.32 (d, J = 1.6Hz, 1H, ArH), 8.85 (d, J = 1.6Hz, 1H, ArH), 8.68 (dd, J = 4.8, 1.6Hz, 1H, ArH), 8.62 (s ,1H,CONH),8.55(d,J=5.3Hz,1H,ArH),8.38(dt,J=8.0,1.9,1.9Hz,1H,ArH),8.10(t,J=1.8Hz,1H,ArH )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com