A method for purifying and oxidizing disulfide bond-containing polypeptides
A disulfide bond and oxidant technology, applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low purity, many impurities in solid phase oxidation, time-consuming and labor-intensive, etc., to improve yield and purity, and operate Simple, solve the effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Octreotide crude peptide purification
[0037] Dissolve and filter 2.0 g of octreotide linear crude peptide, and collect the filtrate for subsequent use.
[0038] Chromatographic column: octadecyl bonded silica gel chromatographic column, the column specification is 5cm×25cm.
[0039] Preparation Process:
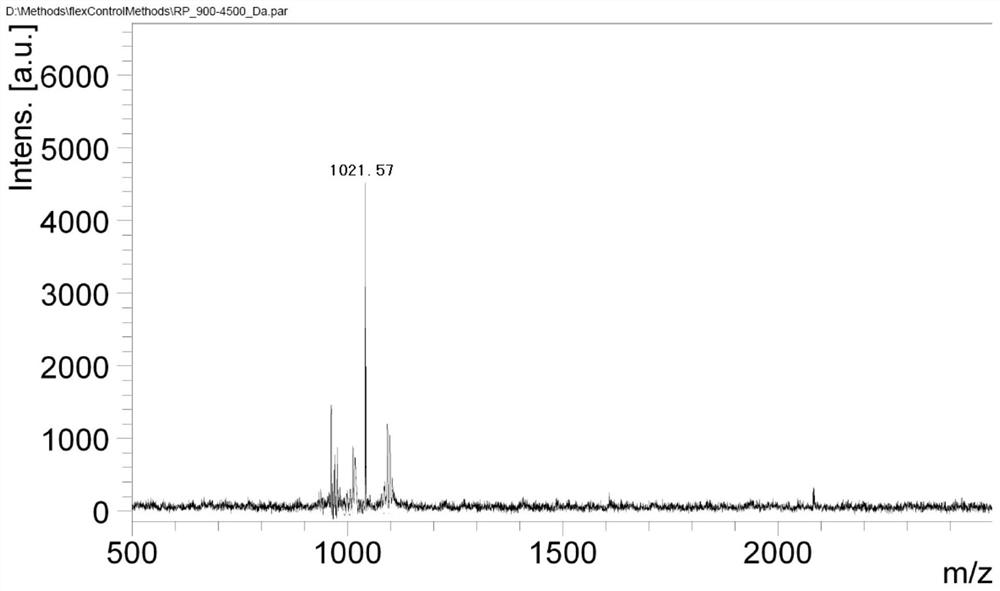
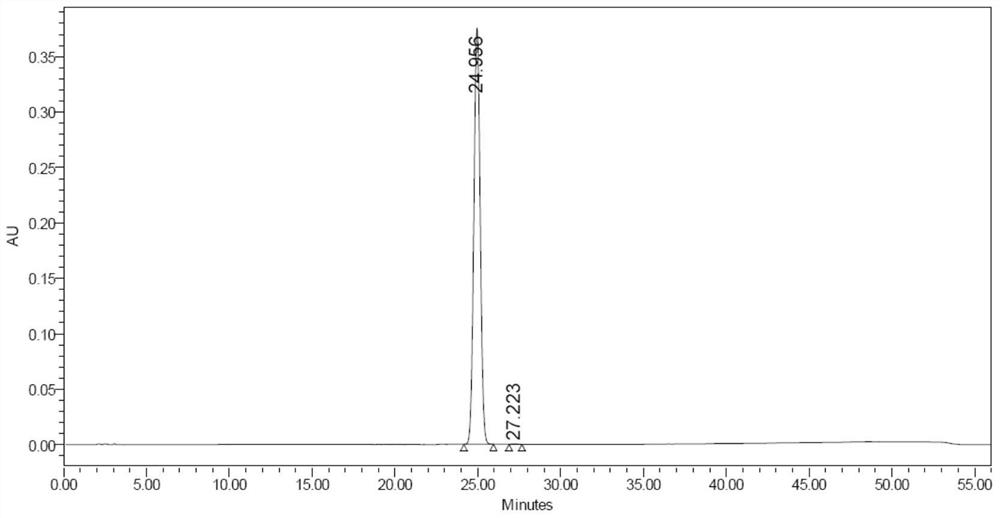
[0040] Adjust the pH of 100 mmol / L potassium dihydrogen phosphate solution to 8.5 with ammonia water as mobile phase A, add 10 g of hydrogen peroxide to phase A, and mix well; use chromatographically pure methanol as phase B. With a flow rate of 60-80ml / min, the detection wave is 230nm. Phase B elution gradient: Gradient elution is performed on the sample with a gradient change of B%: 15%-35%, 50-70min. Purification is complete, while oxidation is complete.
[0041] The oxidized and purified polypeptide obtained in the above step is formed into a stable salt, and octreotide meeting the standard with a purity greater than 99.0% can be obtained after ...
Embodiment 2
[0043] Embodiment 2: Octreotide crude peptide purification
[0044] Dissolve and filter 15 g of octreotide crude peptide, and collect the filtrate for subsequent use.
[0045] 1 Chromatographic column: octadecyl bonded silica gel chromatographic column, the column specification is 10cm×25cm.
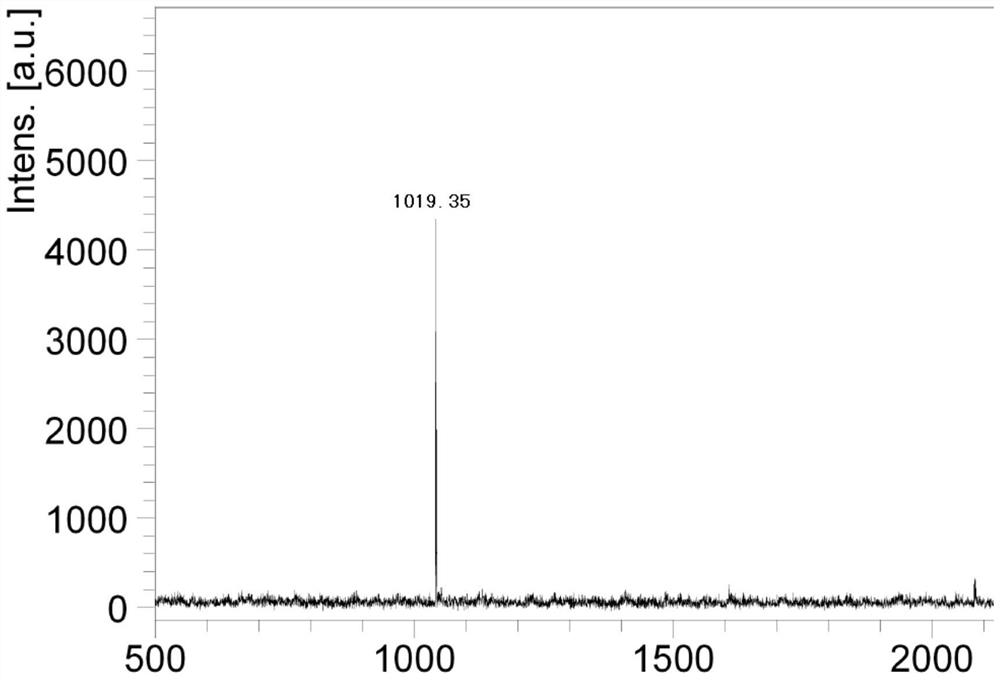
[0046] Adjust the pH of 100 mmol / L sodium dihydrogen phosphate solution to 8.0 with ammonia water as mobile phase A, and add 150 g of hydrogen peroxide; chromatographically pure methanol as phase B. Flow rate: 200ml / min. Detection wavelength: 230nm. Phase B elution gradient: Gradient elution is performed on the sample with a gradient change of B%: 8%-20%, 50-70min. Purification is complete, while oxidation is complete.
[0047] The oxidized and purified polypeptide obtained in the above step is formed into a stable salt, and octreotide meeting the standard with a purity greater than 99.0% can be obtained after freeze-drying.
[0048] After lyophilization, 6.1 g of white powdery soli...
Embodiment 3
[0049] Embodiment 3: Octreotide crude peptide purification
[0050] Dissolve and filter 25 g of octreotide crude peptide, and collect the filtrate for subsequent use.
[0051] Chromatographic column: octadecyl bonded silica gel chromatographic column, the column specification is 15cm×25cm.
[0052] Adjust the pH of 100mmol / L sodium dihydrogen phosphate solution to 7.5 with ammonia water, and add 150g hydrogen peroxide as phase A; chromatographically pure methanol as phase B. Flow rate: 200ml / min. Detection wavelength: 230nm. Phase B elution gradient: Gradient elution is performed on the sample with a gradient change of B%: 8%-20%, 50-70min. Purification is complete, while oxidation is complete.
[0053] The oxidized and purified polypeptide obtained in the above step is formed into a stable salt, and octreotide meeting the standard with a purity greater than 99.0% can be obtained after freeze-drying.
[0054] After lyophilization, 8.9 g of white powdery solid refined pept...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com