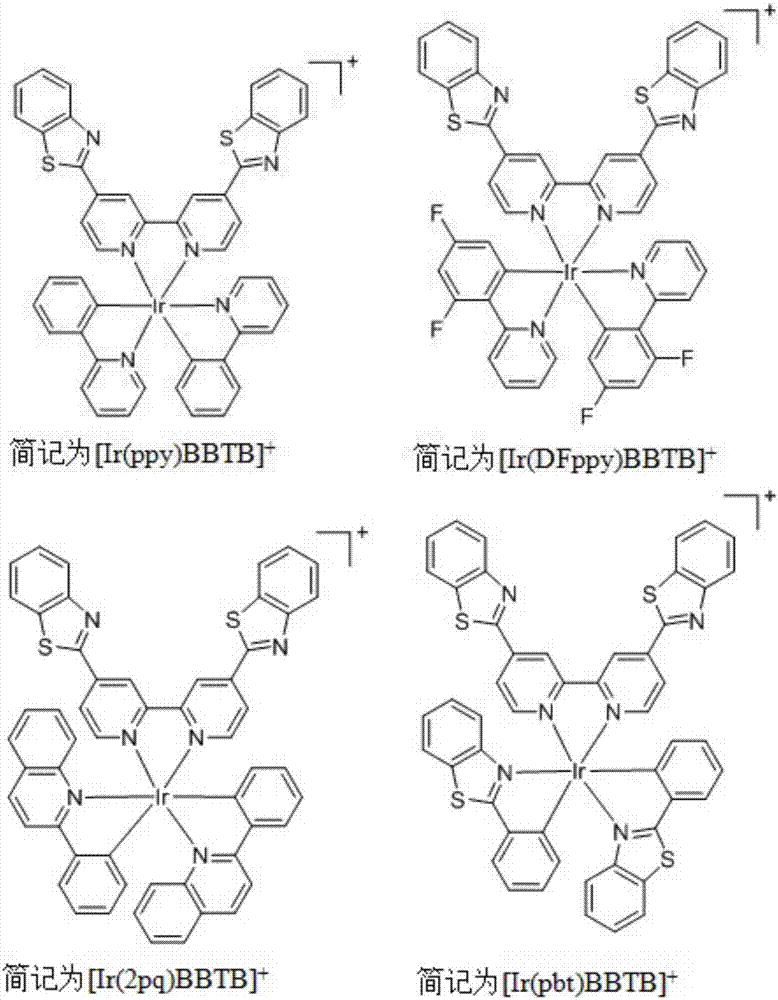
Cell oncosis induction iridium complexes, and preparation method and antitumor application thereof
An iridium complex and reaction technology, applied in the field of biomedicine, can solve the problems of cell apoptosis and other problems, and achieve the effects of strong growth inhibition ability, good inducing activity and small lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The synthesis of embodiment 1 cyclometalation Ir (III) complexes
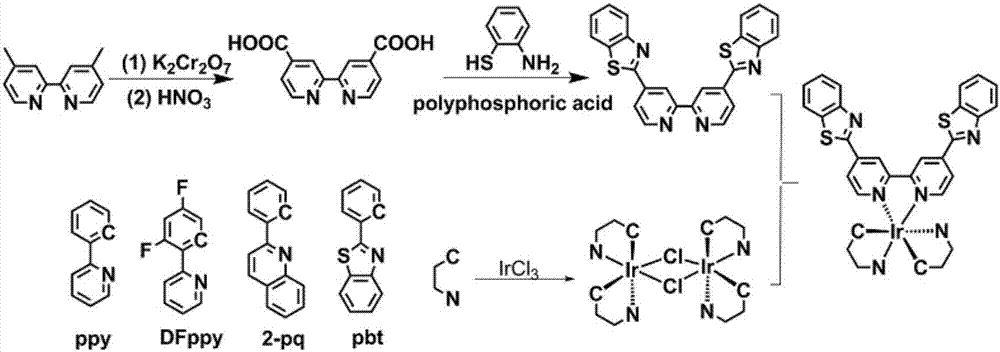
[0085] The synthetic approach of the cyclometalated Ir(III) complex of the present invention is as follows figure 2 As shown, the specific method is as follows:
[0086] (1) The synthesis method of the main ligand 4,4'-dibenzothiazole-2,2'-bipyridine (BBTB):
[0087] 1) Synthesis of 4,4'-dicarboxy-2,2'-bipyridine (BCB):
[0088] First, dissolve 4,4'-dimethyl-2,2'-bipyridine (1.0g) in 50mL of concentrated sulfuric acid, slowly add 5.0g of potassium dichromate solid to oxidize while maintaining the temperature, and the reaction produces a dark green mixed liquid. The reaction solution was poured into 80 mL of ice water to produce a light yellow precipitate, which was filtered and washed with water, and the solid was placed in 30 mL of 50% nitric acid for reflux reaction for 4 hours. Pour the reaction solution into ice cubes and add 800mL of water to dilute, filter with suction to obtain a large amount...
Embodiment 2
[0099] Example 2 Cell Subcellular Organelle Targeting Experiment of Cyclometallated Ir(III) Complexes
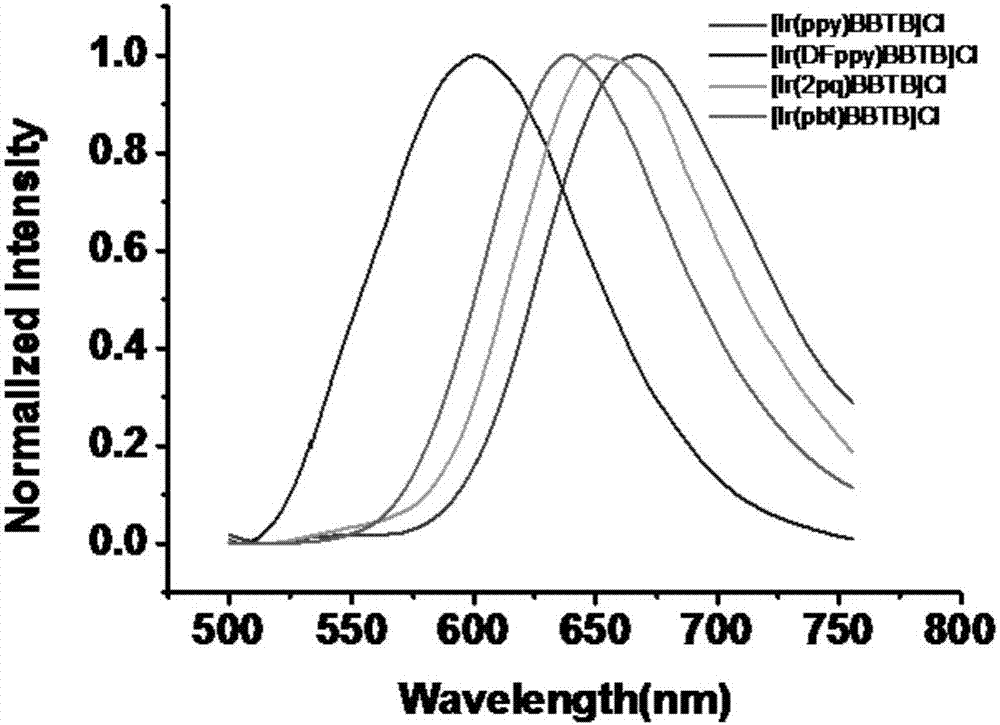
[0100] 1. After the cyclometalated iridium (III) complex of the present invention absorbs light of 380nm to 405nm and is irradiated, electrons transition from the ground state to 3 The MLCT orbital is in an excited state, and then emits phosphorescence during the transition back to the ground state (eg image 3 shown).
[0101] 2. In addition, using this property, the distribution state of the complex in the cell can be observed by laser confocal microscopy. Co-staining of this series of complexes with the commercial mitochondrial dye MitoTracker Green FM showed that the series of complexes ([Ir(ppy)BBTB]Cl, [Ir(DFppy)BBTB]Cl, [Ir(2pq)BBTB]Cl, [Ir (pbt)BBTB]Cl) is mainly located in the mitochondria of cells, and its colocalization coefficients (Pearson's Colocalization Coefficients) are 0.83, 0.85, 0.92, 0.87 respectively, and the coincidence rate is relatively high (such ...
Embodiment 3
[0103] Example 3 Cytotoxicity MTT experiment of cyclometalated Ir(III) complexes
[0104] 1. Experimental method
[0105] (1) Experimental samples:
[0106] Ir(III) complexes, the control is the commonly used anti-tumor complex cisplatin CDDP (Pt(NH 3 ) 2 Cl 2 ).
[0107] (2) method
[0108] Tumor or normal cells in the logarithmic growth phase were taken, and the cell density was adjusted to 5×10 3 Each sample / ml was inoculated in a 96-well culture plate, and a total of 5 concentrations were added to each sample in the experiment by logarithmic dilution. Three replicate holes were set for each concentration, and more than eight replicate holes were set for the control.
[0109] Experimental samples were dissolved with DMSO and diluted with DMEM culture medium. After 24 hours of attachment to the wall, add the sample, and still place the cells at 37°C, 5% CO 2 Continue culturing in the incubator for 48 hours, then add MTT, continue culturing for another 4 hours, absor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com