Pharmaceutical composition and kit for treating cancers
A technology of composition and kit, applied in the direction of drug combination, drug delivery, and medical raw materials derived from mammals, etc., can solve the problem of lack of effective means for advanced refractory solid tumors or hematological malignancies, and achieve defense against autoimmunity Escape mechanism, good application prospects, and the effect of improving clinical efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
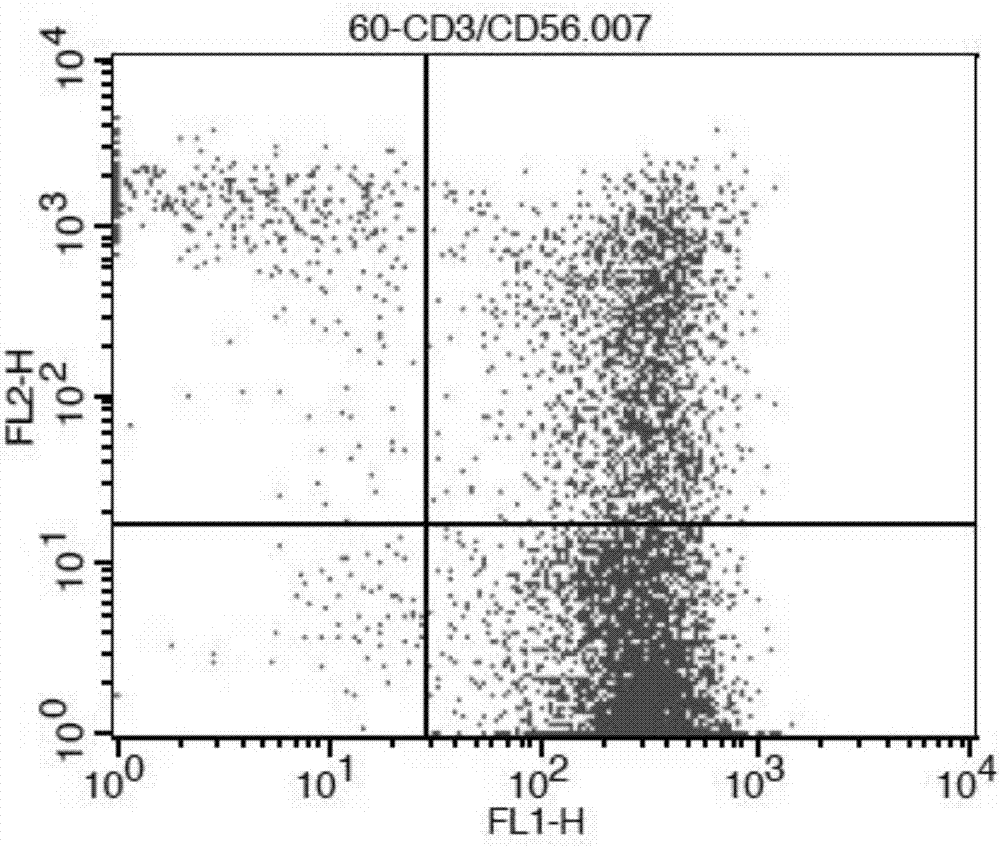
[0045] Example 1 Acquisition of DC-CIK cells
[0046] 1. Separation of mononuclear cells from peripheral blood samples: take fresh human peripheral blood samples, add blood anticoagulants to them to prepare anticoagulant blood samples; add 1 / 3 to 2 / 3 volume of normal saline to the anticoagulant blood samples , mix thoroughly, separate the mononuclear cells in the resulting mixture with 1 to 1.5 times the volume of lymphocyte separation medium (Tianjin Haoyang, product number LTS1077006), and use lymphocyte culture medium GT-T551 (Takara, product number: DL-104 ) adjust the monocyte density to 1×10 6 ~5×10 6 cells / mL to obtain a suspension of peripheral blood mononuclear cells; wash the suspension of peripheral blood mononuclear cells with normal saline, centrifuge at room temperature for 20-30 min at a speed of 1500-2000 r / min, and obtain buffy coat cells.
[0047] 2. Separation of DC and CIK cells: Inoculate monocytes to 75cm 2 2 hours after the flask was cultured, the sus...
Embodiment 2
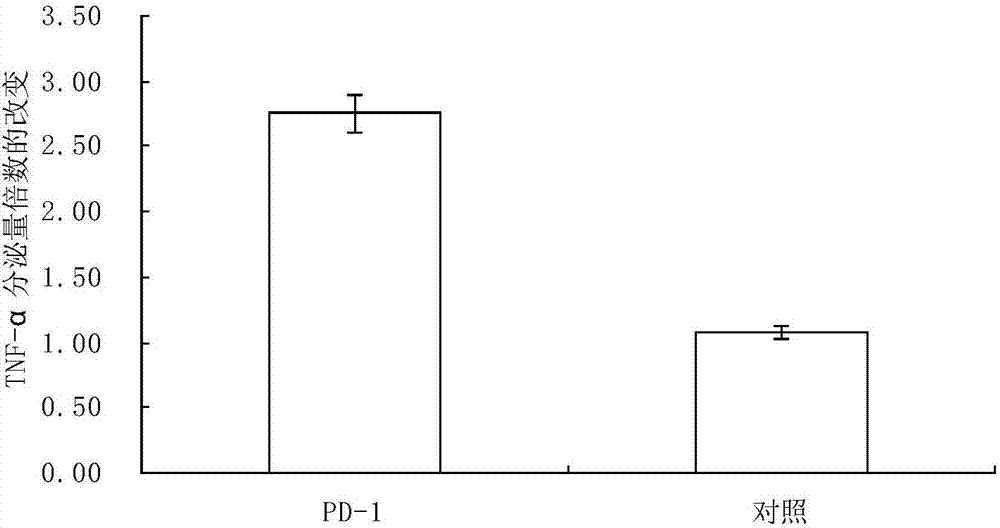
[0049] Example 2 Anti-PD-1 antibody activation of CIK cells in vitro experiment CIK cells stimulated by CD3 monoclonal antibody for 5 days were taken (CIK cells were cultured alone, and the immune checkpoint of CIK cells in an activated state was blocked by PD-1 antibody at this time). Broken) into 96-well flat-bottom plate, after incubation overnight, add 10μg / ml anti-PD-1 antibody and 100ng / ml tetanus toxin (TT), the control group did not add PD-1 antibody, and collected after 3 days of culture The supernatant was used to detect the secretion level of TNF-α in the PD-1 antibody group and the control group with the TNF-α ELISA detection kit from Thermofisher. Results (see figure 2 ) shows that the anti-PD-1 antibody can stimulate the function of CIK cells, and the increase rates of TNF-α secretion in the control group and the anti-PD-1 antibody group are 107.3% and 275.4%, respectively, with significant difference, p<0.01.
Embodiment 3
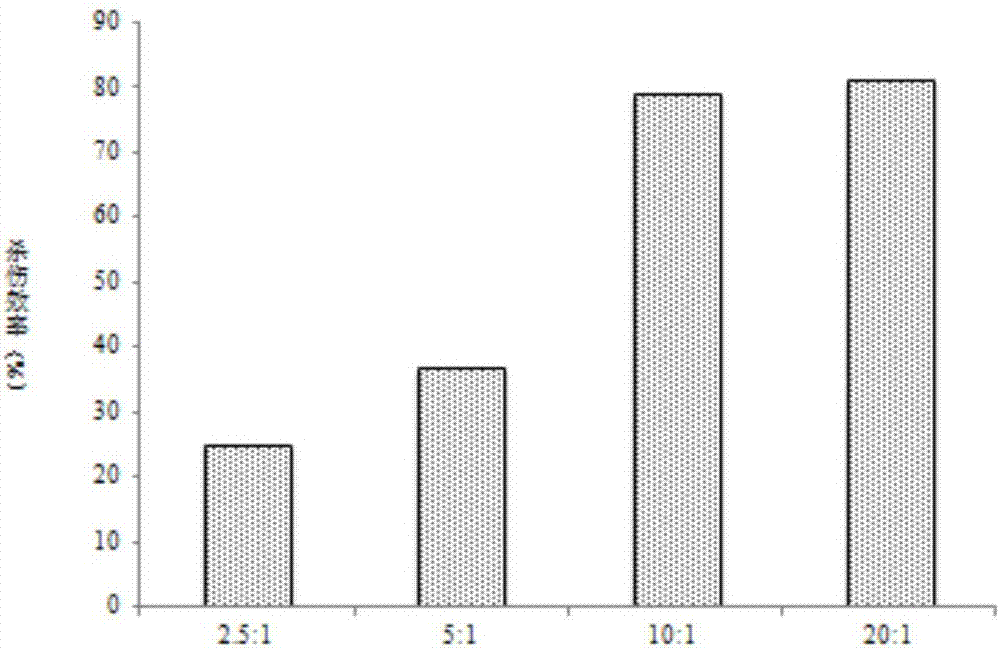
[0050] MTT assay of the cytotoxic effect of embodiment 3DC-CIK cells on tumor cells
[0051] The DC-CIK co-culture activated by PD-1 antibody cultured for 12 days was used as the effector cells, and the A549 lung cancer cell line was used as the target cells. Take the A549 cells in the logarithmic growth phase, and adjust the cell concentration of DC-CIK to 2.5×10 5 / mL, 5×10 5 / mL, 1×10 6 / mL, 2×10 6 / mL, the number ratio of effector cells to target cells was 2.5:1, 5:1, 10:1, 20:1. The experiment was divided into 3 groups, with 3 replicate wells in each group. The experimental group added 100 μL / well of effector cells and target cells, the target cell group added 100 μL / well of target cells and culture medium, and the effector cell group added effector cells and culture medium 100 μL / well. 100 μL / well, set at 37°C, 5% CO 2 Incubate in an incubator for 24 hours, add MTT reagent, 20 μL / well, incubate at 37°C for 4 hours, then add washing reagent 100 μL / well, incubate at 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com