A kind of n-alkylhydroxamic acid-dithiocarbamate collector, its preparation and application
A technology of dithiocarbamate and alkylhydroxamic acid, which is applied in the field of metal ore flotation, and can solve the problem of N-alkylhydroxamic acid-dithiocarbamate compounds, etc. problems, achieve good flotation performance, wide application range, and enhance the effect of adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
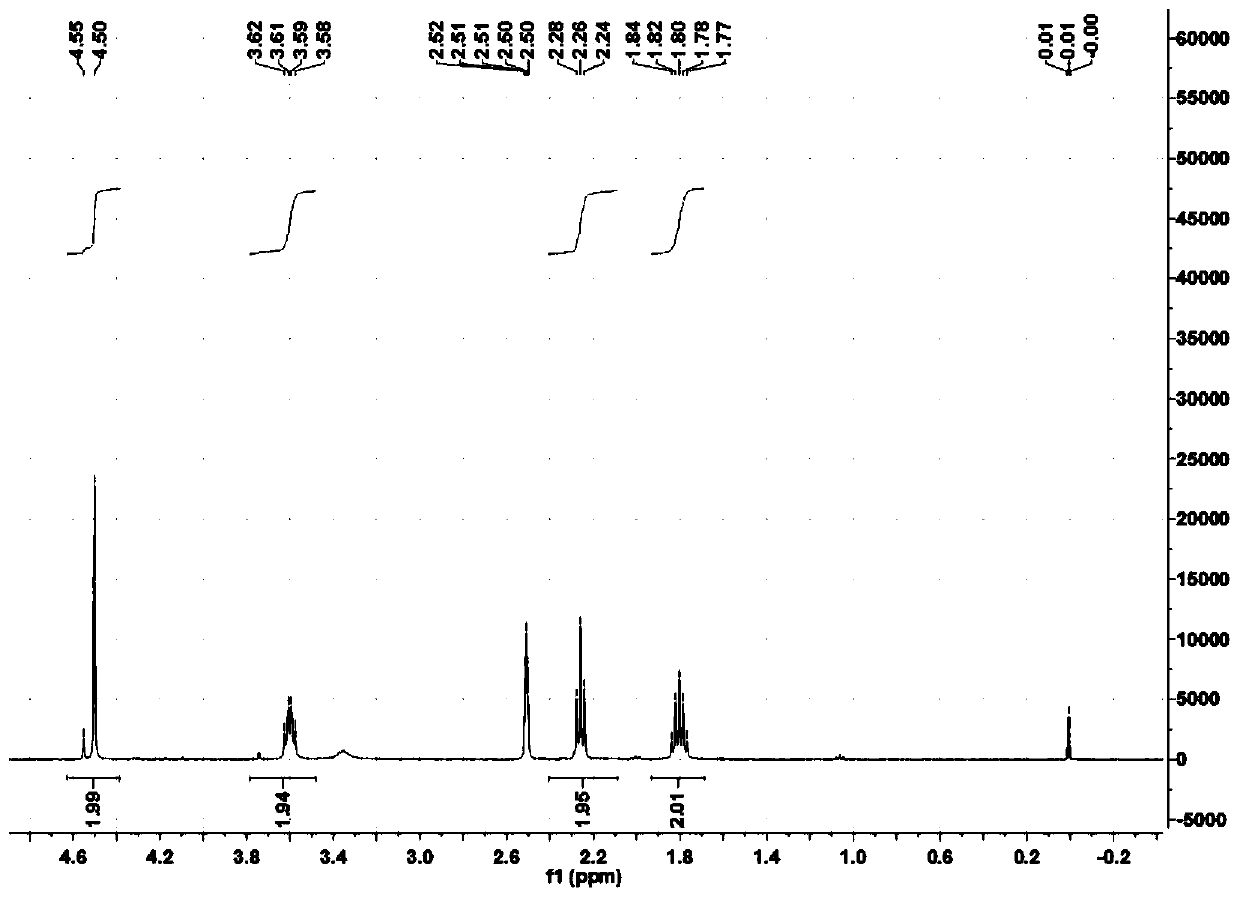
[0065] Example 1 Preparation of N-(4-butyl hydroxamic acid)-S-n-octyl dithiocarbamate
[0066] Slowly add 9.14 parts of carbon disulfide to the mixed solution of 12.98 parts of 4-aminobutyl hydroxamic acid and 18 parts of methanol under stirring, keep the temperature of the reaction solution below 10°C during the dropping process, and stir at 10°C after the addition After reacting for 4 hours, add 5.42 parts of sodium hydroxide solid particles in batches, then add 19.3 parts of n-octane bromide under stirring at 15°C, and then stir and react at 15°C for 15 hours. Then lower the temperature to below 5°C, add concentrated sulfuric acid to acidify to a pH of about 4. Filtration, the filtrate was distilled under reduced pressure, washed with water, and dried to obtain a white solid, which was N-(4-butylhydroxamic acid)-S-n-octyl dithiocarbamate, based on bromo-n-octane The yield was 88.6%.
Embodiment 2
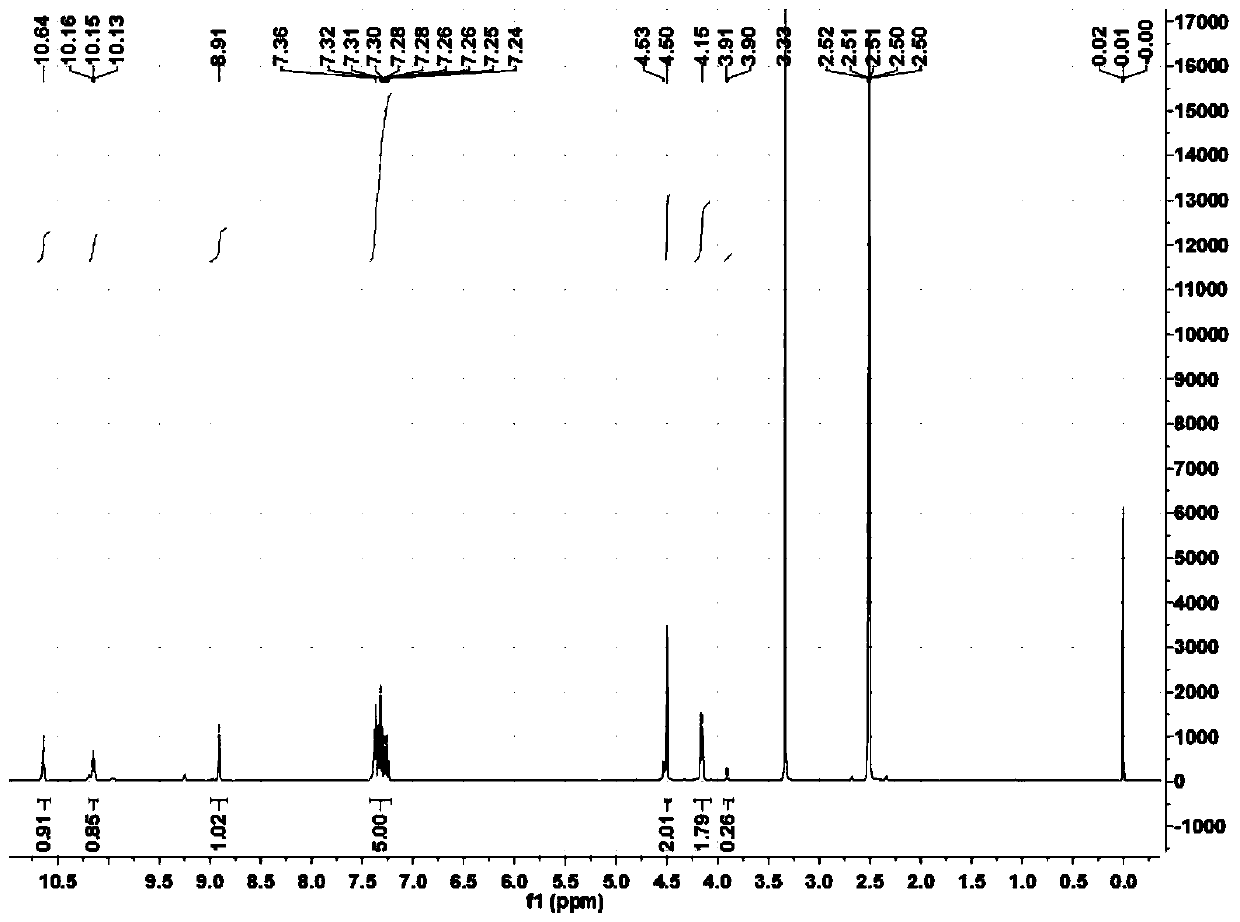
[0067] Example 2 Preparation of N-(6-hexyl hydroxamic acid)-S-dodecyl dithiocarbamate
[0068] Slowly add 9.14 parts of carbon disulfide to the mixed solution of 16.06 parts of 6-aminohexylhydroxamic acid and 18 parts of methanol under stirring, keep the temperature of the reaction solution below 10°C during the dropping process, and stir the reaction at 10°C after the addition After 4 hours, add 5.42 parts of sodium hydroxide solid particles in batches, then add 24.9 parts of dodecane bromide under stirring at 20°C, and then stir and react at 20°C for 15 hours. Then lower the temperature to below 5°C, add concentrated sulfuric acid to acidify to a pH of about 4. Filtration, the filtrate was distilled under reduced pressure, washed with water, and dried to obtain a white solid, which was N-(6-hexylhydroxamic acid)-S-dodecyl dithiocarbamate, based on bromododecane The yield was 89.3%.
Embodiment 3
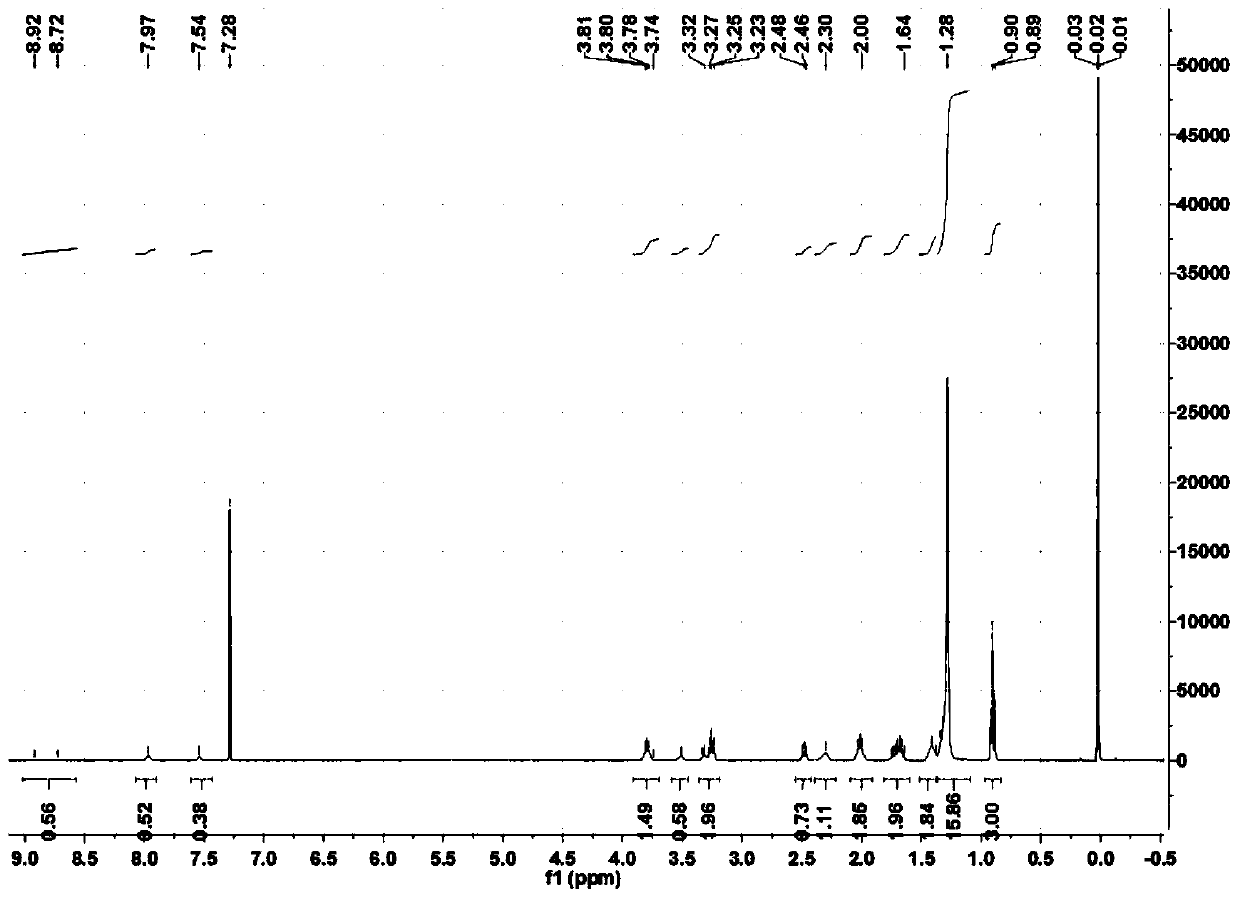
[0069] Example 3 Preparation of N-(2-ethylhydroxamic acid)-S-benzyl dithiocarbamate
[0070] Add 9.0 parts of 2-aminoglyoxamic acid to a mixed solution of 12.7 parts of benzyl chloride, 8.36 parts of carbon disulfide, and 30 parts of PEG-200 under stirring at 20°C, and then stir and react at 20°C for 2 hours. After the reaction, the temperature was lowered to below 5°C, and then 100 parts of water were added to the reaction flask to obtain a white solid, which was N-(2-ethylhydroxamic acid)-S-benzyl dithiocarbamate. The yield based on benzyl chloride was 82.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com