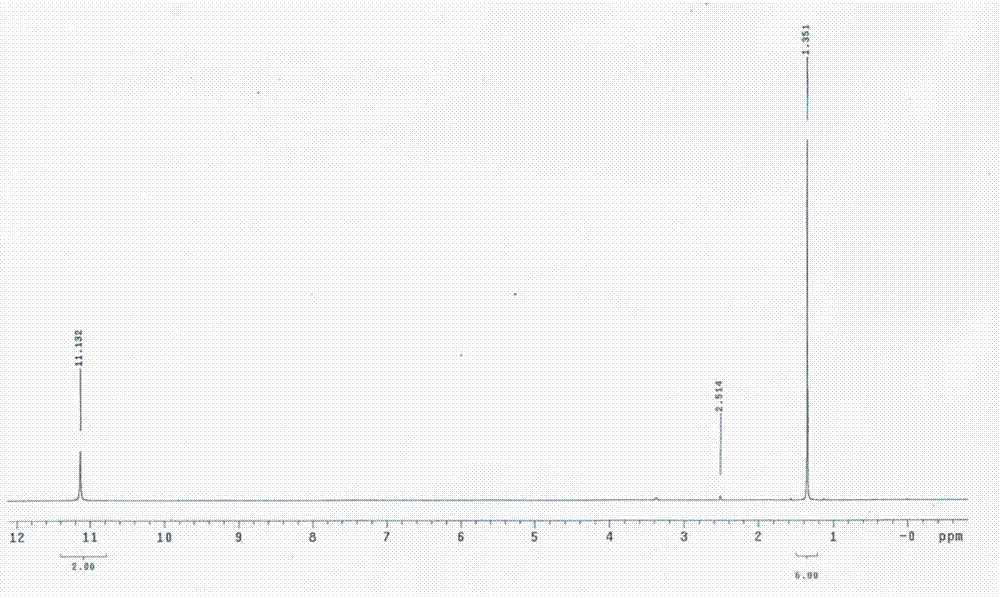
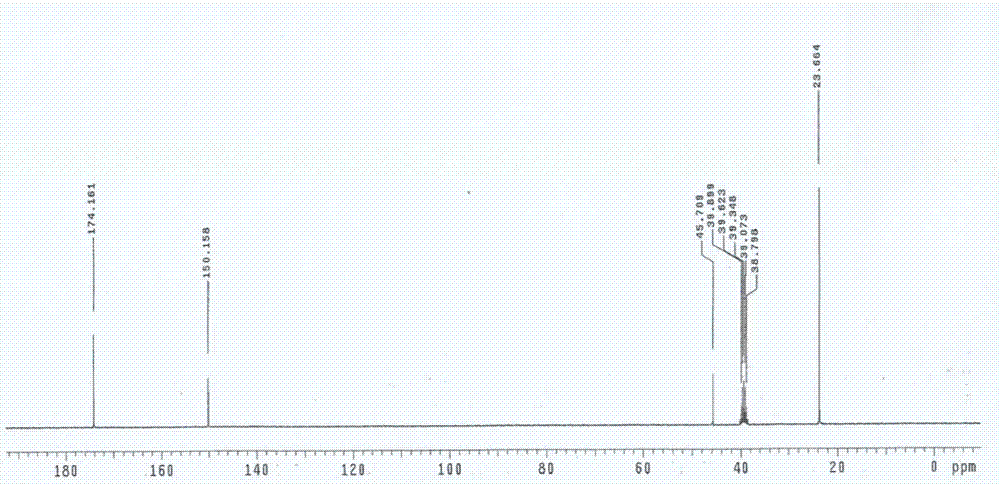
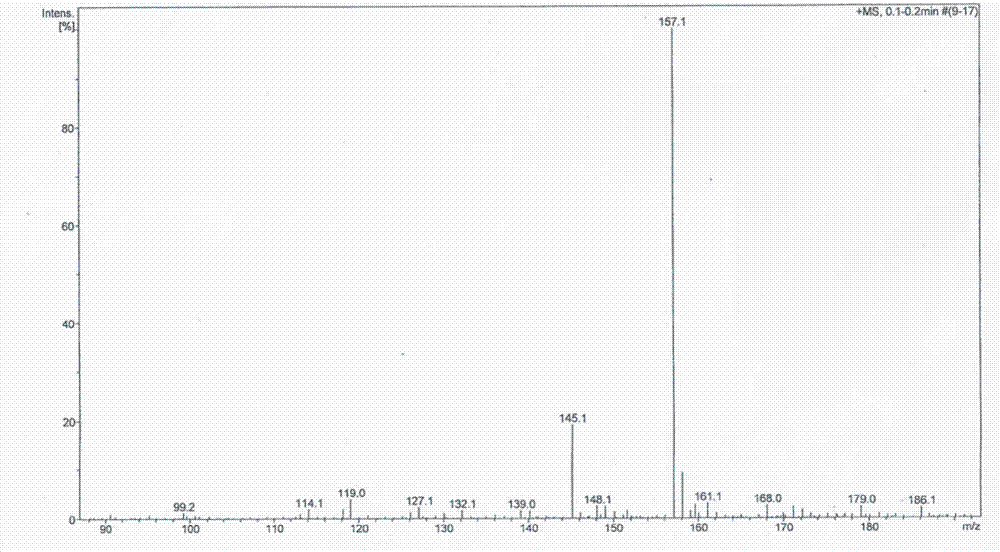
Preparation method for 5,5-dimethylbarbituric acid as impurity of butalbital
A technology of dimethyl barbituric acid and butalbital, which is applied in the field of drug synthesis to achieve the effects of simple post-processing, easy availability of raw materials, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of 2,2-dimethylmalonate diethyl ester
[0026] Add sodium ethoxide (101.9g, 1.5mol) into absolute ethanol (750mL), stir until completely dissolved, add diethyl methylmalonate (174.19g, 1mol) dropwise at 20°C, and drop it within 1h After that, keep stirring at 20°C for 0.5h. Then continue to add iodomethane (156.1 g, 1.1 mol) dropwise at 20°C, and control the dropwise completion within 1 h. After that, the temperature was raised to 80 degrees, and the temperature was kept at reflux for 10 hours. Heating was stopped, all the ethanol was distilled off under reduced pressure, and then 800 ml of distilled water was added to the residue to dissolve it. After dissolving, it was extracted twice with 2*800ml ethyl acetate, the ethyl acetate layers were combined, dried with anhydrous sodium sulfate, filtered with suction, and the ethyl acetate was concentrated to obtain 127.6g of light yellow oil. The purity of the crude product is 92.8%, and the yield is 62.89...
Embodiment 2
[0033] (1) Preparation of 2,2-dimethylmalonate diethyl ester
[0034] Add sodium ethoxide (122.3g, 1.5mol) to absolute ethanol (850mL), stir until completely dissolved, add diethyl methylmalonate (174.19g, 1mol) dropwise at 30°C, and drop it within 1h Complete, then continue to insulate and react at 30 degrees for 1h. Then methyl iodide (170.3 g, 1.2 mol) was added dropwise at 30°C, and the drop was completed within 1 h. After that, the temperature was raised to 80 degrees, and the temperature was kept at reflux for 15 hours. Heating was stopped, all the ethanol was distilled off under reduced pressure, and then 800 ml of distilled water was added to the residue to dissolve it. After dissolving, it was extracted twice with 2*800ml ethyl acetate, the ethyl acetate layers were combined, dried with anhydrous sodium sulfate, filtered with suction, and the ethyl acetate was concentrated to obtain 143.36g of light yellow oil. The purity of the crude product is 98.8%, and the yiel...
Embodiment 3
[0040] (1) Preparation of 2,2-dimethylmalonate diethyl ester
[0041] Add sodium ethoxide (163.1g, 2mol) into absolute ethanol (950mL), stir until completely dissolved, add diethyl methylmalonate (174.19g, 1mol) dropwise at 40°C, and control the dropwise completion within 1h , and then continue to insulate the reaction at 40 degrees for 2h. Then iodomethane (184.5 g, 1.3 mol) was added dropwise at 40°C, and the drop was completed within 1 h. Afterwards, the temperature was raised to 80 degrees, and the temperature was kept at reflux for 20 hours. Heating was stopped, all the ethanol was distilled off under reduced pressure, and then 800 ml of distilled water was added to the residue to dissolve it. After dissolving, extract twice with 2*800ml of ethyl acetate, combine the ethyl acetate layers, dry with anhydrous sodium sulfate, filter with suction, concentrate the ethyl acetate to obtain 145.68g of light yellow oil. The purity of the crude product is 98.9%, and the yield is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com