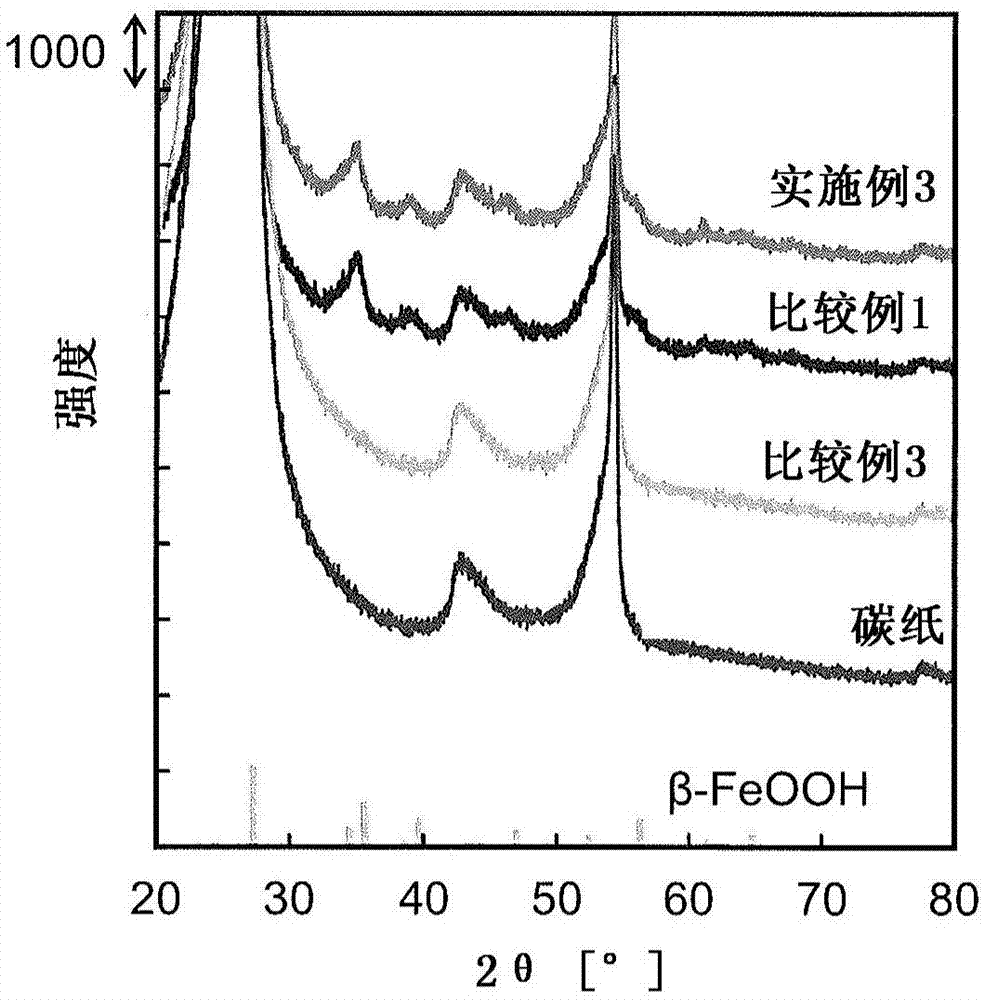
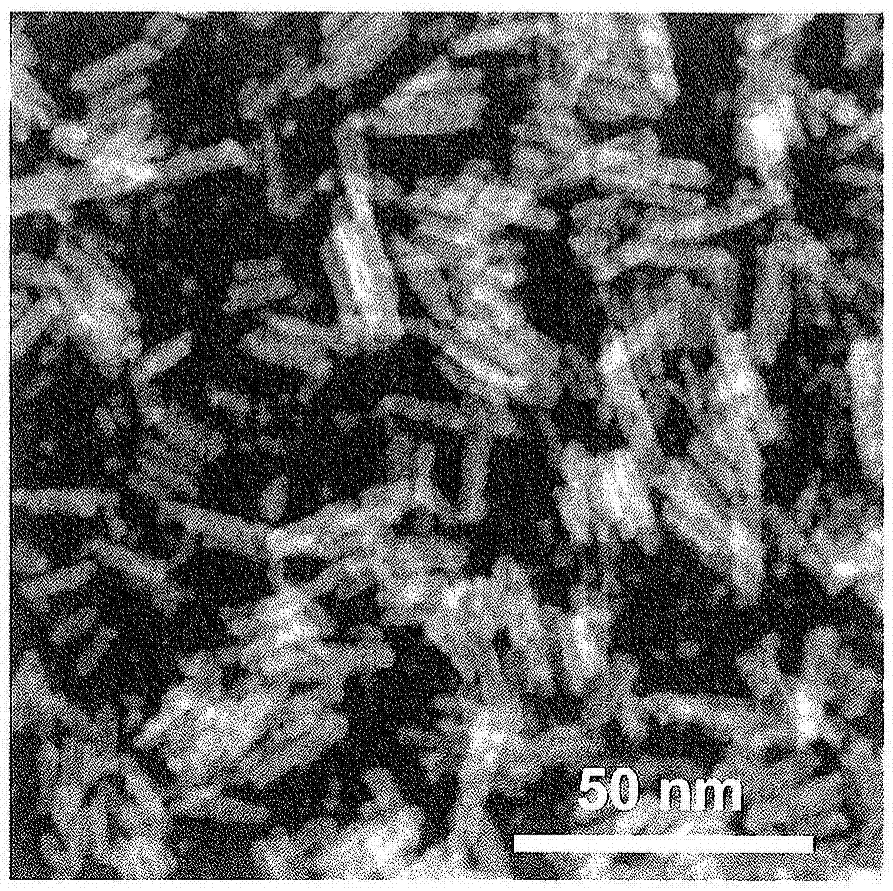
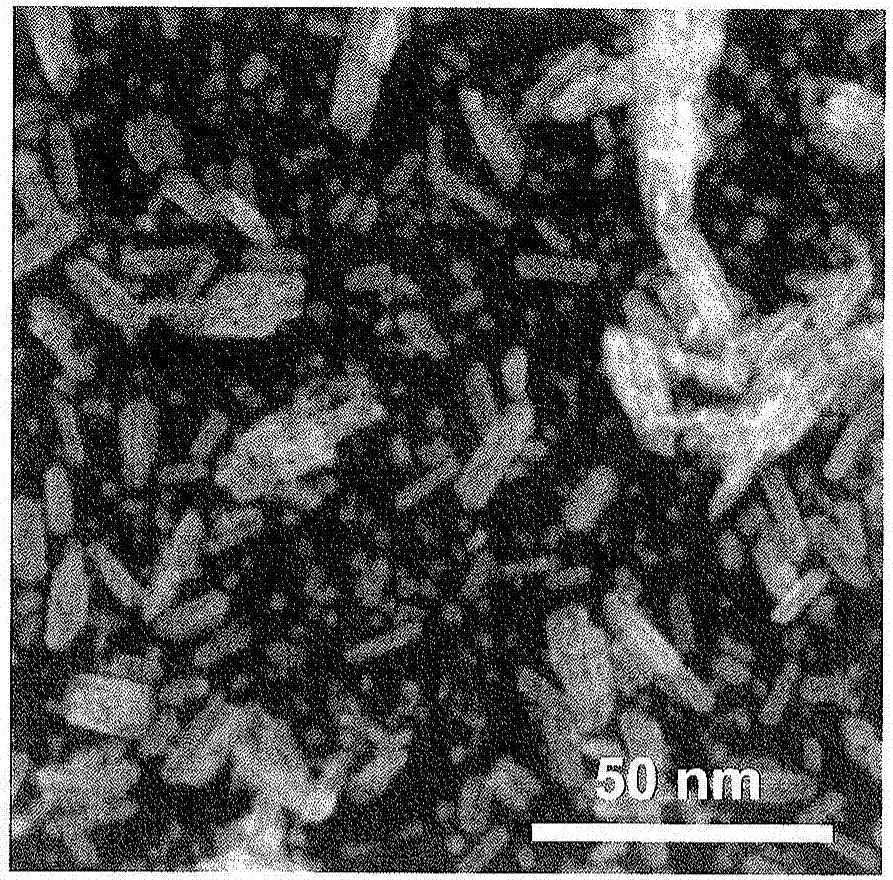
Iron compound particles, method of producing iron compound particles, and oxidation catalyst using iron compound particles
A technology of iron compounds and particles, applied in the field of oxidation catalysts, to achieve the effect of excellent oxidation catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] In a beaker, FeCl 3 ·6H 2 O (27.30g, 101mmol) and Zn (NO 3 ) 2 ·6H 2 O (1.25g, 4.20mmol) was dissolved in ion-exchanged water (500ml). Thus, a raw material aqueous solution containing metal ions (raw material solution A) having an Fe ion concentration of 0.2 mol / L was prepared. Also, in a beaker, an ethylenediamine solution (11 ml) diluted to 1 / 2 with ion-exchanged water was dissolved in ion-exchanged water (500 ml). Thus, a raw material aqueous solution (raw material solution B) containing a neutralizing agent was prepared. Raw material solutions A and B were mixed in a beaker with a magnetic stirrer using a stirring bar (rotational speed: 400 rpm) and stirred together for 30 minutes at room temperature (25° C.). Thus, a colloidal solution of an iron compound was prepared. By using a pH meter, the pH of the resulting colloid solution was measured and found to be 2.2.
Embodiment 2
[0082] The colloidal solution of iron compound was prepared in the same manner as in Example 1, except that Co(NO 3 ) 2 ·6H 2 O (1.22g, 4.19mmol) instead of Zn(NO 3 ) 2 ·6H 2 O. The pH of the obtained colloidal solution was 2.2.
Embodiment 3
[0084] The colloidal solution of iron compound is prepared in the same manner as in Example 1, except that Ni(NO 3 ) 2 ·6H 2 O (1.21g, 4.16mmol) instead of Zn(NO 3 ) 2 ·6H 2 O. The pH of the obtained colloidal solution was 2.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Crystallite diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com