Preparation method of compound preparation containing metformin hydrochloride and vildagliptin
A technology of metformin hydrochloride and compound preparations, which is applied to medical preparations containing active ingredients, pill delivery, and pharmaceutical formulations, etc., which can solve problems such as uneven content, achieve low energy consumption, low production equipment costs, and solve tablet content. uneven effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
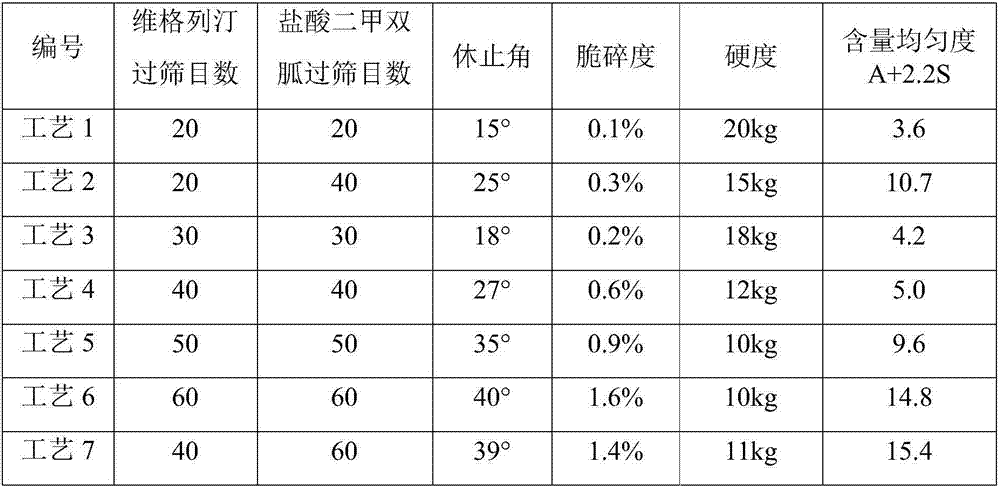
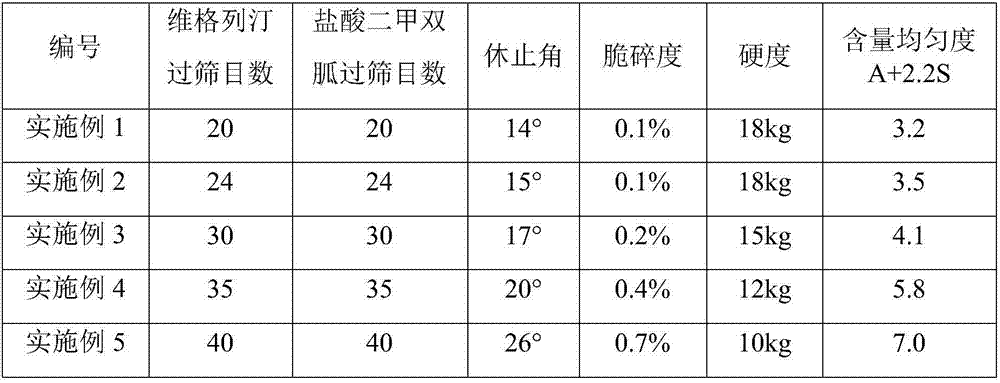
[0048] components Dosage (mg / tablet) Vildagliptin 50 Metformin Hydrochloride 1000 Hydroxypropyl Cellulose 80.5 Magnesium stearate 12 Sheet weight 1142.5
[0049] (1) Weigh vildagliptin (1000g) and hydroxypropyl cellulose (the weight of hydroxypropyl cellulose is 1% of vildagliptin, 10g), mix uniformly in equal increments, add 95% ethanol to prepare soft material, granulated through a 20-mesh sieve, dried at 40°C, and granulated through a 20-mesh sieve.
[0050](2) Take by weighing metformin hydrochloride (1000g) and hydroxypropyl cellulose (the weight of hydroxypropyl cellulose is 8% of vildagliptin, 80g), equal increase and mix uniformly, add water to prepare soft material, cross 20 Granulate with a mesh sieve, dry at 60°C, pass through a 20-mesh sieve for granulation.
[0051] (3) Take by weighing one-twentieth (50.5g) of the granule weight in step (1), mix it with the granule obtained in step (2) and magnesium stearate (12g), an...
Embodiment 2
[0053] components Dosage (mg / tablet) Vildagliptin 50 Metformin Hydrochloride 850 Hydroxypropyl Cellulose 100 Magnesium stearate 12 Sheet weight 1012
[0054] (1) Weigh vildagliptin (500g) and hydroxypropyl cellulose (the weight of hydroxypropyl cellulose is 2% of vildagliptin, 10g), mix uniformly in equal increments, add 95% ethanol to prepare soft material, granulated through a 24-mesh sieve, dried at 40°C, and granulated through a 24-mesh sieve.
[0055] (2) Take by weighing metformin hydrochloride (850g) and hydroxypropyl cellulose (the weight of hydroxypropyl cellulose is 11.6% of vildagliptin, 99g), equal increase and mix uniformly, add water to prepare soft material, pass 24 Granulate with a mesh sieve, dry at 60°C, pass through a 24-mesh sieve for granulation.
[0056] (3) Take by weighing one-tenth (51g) of the granule weight in step (1), mix it with the granule obtained in step (2) and magnesium stearate (12g), and press i...
Embodiment 3
[0058] components Dosage (mg / tablet) Vildagliptin 50 Metformin Hydrochloride 1000 Hydroxypropyl Cellulose 120 Magnesium stearate 12 Sheet weight 1182
[0059] (1) Weigh vildagliptin (1000g) and hydroxypropyl cellulose (the weight of hydroxypropyl cellulose is 4% of vildagliptin, 40g), mix uniformly in equal increments, add 95% ethanol to prepare soft material, granulated through a 30-mesh sieve, dried at 40°C, and granulated through a 30-mesh sieve.
[0060] (2) Take by weighing metformin hydrochloride (1000g) and hydroxypropyl cellulose (the weight of hydroxypropyl cellulose is 11.8% of vildagliptin, 118g), equal increase and mix uniformly, add water to prepare soft material, cross 30 Granulate with a mesh sieve, dry at 60°C, pass through a 30-mesh sieve for granulation.
[0061] (3) Take by weighing one-twentieth (52g) of the granule weight in step (1), mix it with the granule obtained in step (2) and magnesium stearate (12g), an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com