Fexofenadine hydrochloride dry suspension, and preparation method thereof
A technology of fexofenadine hydrochloride and dry suspension, which is applied in the field of pharmaceutical preparations and can solve problems such as poor solubility, slow dissolution, and poor uniformity of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
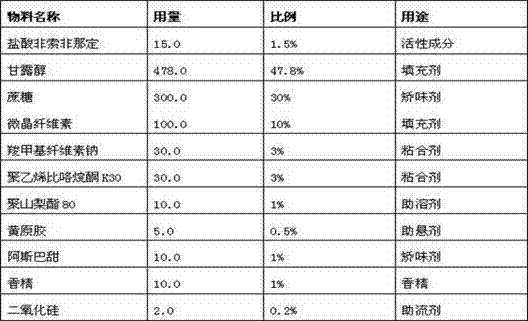
[0020] Prepare fexofenadine hydrochloride dry suspension, adopt the following prescription: (unit: g)
[0021]
[0022] And adopt following preparation method to prepare:
[0023] 1. Weigh fexofenadine hydrochloride, polysorbate 80 and polyvinylpyrrolidone K30 according to the prescription amount, add 95% ethanol in the prescription amount, stir and dissolve to obtain a drug-containing solution for later use;
[0024] 2. Weigh mannitol, sucrose, microcrystalline cellulose and sodium carboxymethyl cellulose according to the prescription amount, place them in the top spray device of the multi-functional granulator coating machine, turn on the equipment, add the drug-containing solution obtained in step 1 to carry out Top-spray granulation, the obtained granules are passed through a 24-mesh sieve for granulation, and then mixed evenly with the added xanthan gum, aspartame, essence and silicon dioxide, and then packaged separately.
Embodiment 2
[0028] Prepare fexofenadine hydrochloride dry suspension, adopt following prescription:
[0029] Material name
[0030] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com