Two enantiomers of 2,6-diamido triptycene and detection and separation method thereof
A technology of diaminotriptycene and enantiomers, which is applied in the field of two enantiomers of 2,6-diaminotriptycene and its detection and separation, can solve problems that have not been seen in research reports so far
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Analysis and determination of 2,6-diaminotriptycene enantiomers
[0044] Instrument and chromatographic conditions:
[0045] High-performance liquid chromatography, the chromatographic column is Chiralpak IC, 0.46cm I.D.×15cm L, 1.0μL; the mobile phase is absolute ethanol: diethylamine=100:0.1 (V / V); the column temperature is 35°C; The flow rate is 1.0mL / min; the detection wavelength is 254nm.
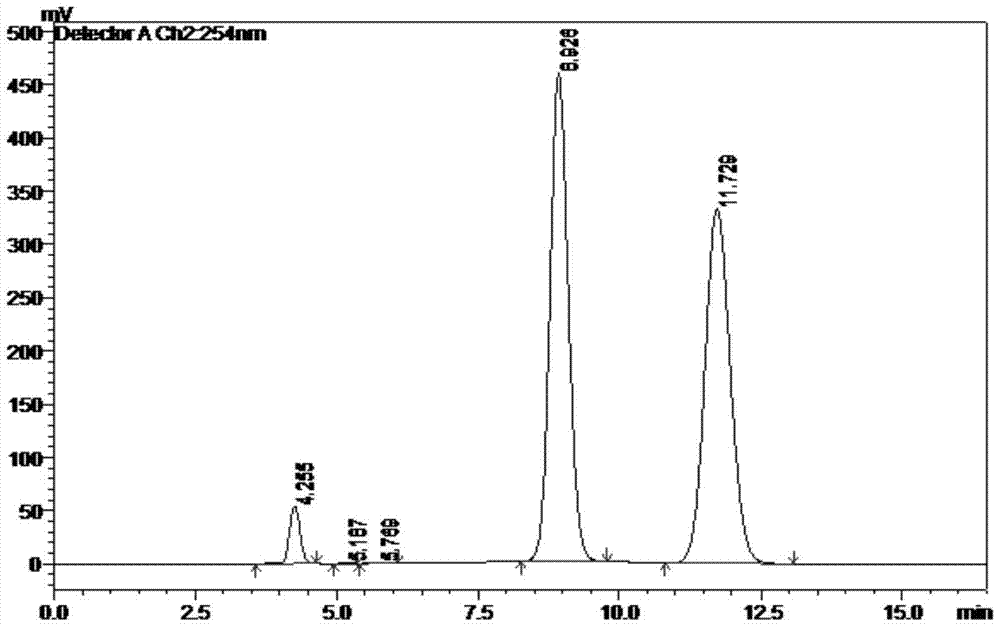
[0046] Sample preparation: take 2,6-diaminotriptycene racemate, add mobile phase to dissolve and dilute, prepare a solution with a solubility of 1 mg / mL, shake well as the sample to be tested, inject it into a high-performance liquid chromatograph, flow Phase elution, according to the peak time of the chromatogram, record the chromatogram, the separation degree of the two main peak components is greater than 2.0, see the attached figure 1 , the peak data in the figure are shown in Table 1: the main peak No. 4 represents 2,6-diaminotriptycene in (S,S) configuration, ...
Embodiment 2
[0049] Example 2: 2,6-diaminotriptycene enantiomer (9S,10S)-9,10-dihydro-9,10-[1,2]benzanthracene-2,6-diamine (Peak 4 components in embodiment 1 separation chromatography) detection
[0050] Instrument and chromatographic conditions:
[0051] High-performance liquid chromatography, the chromatographic column is Chiralpak IC, 0.46cm I.D. × 15cm L, 1.0 μ L; the mobile phase is ethanol: diethylamine = 100: 0.1 (V / V); the column temperature is 35 ° C; the flow rate is 1.0mL / min; detection wavelength is 254nm.
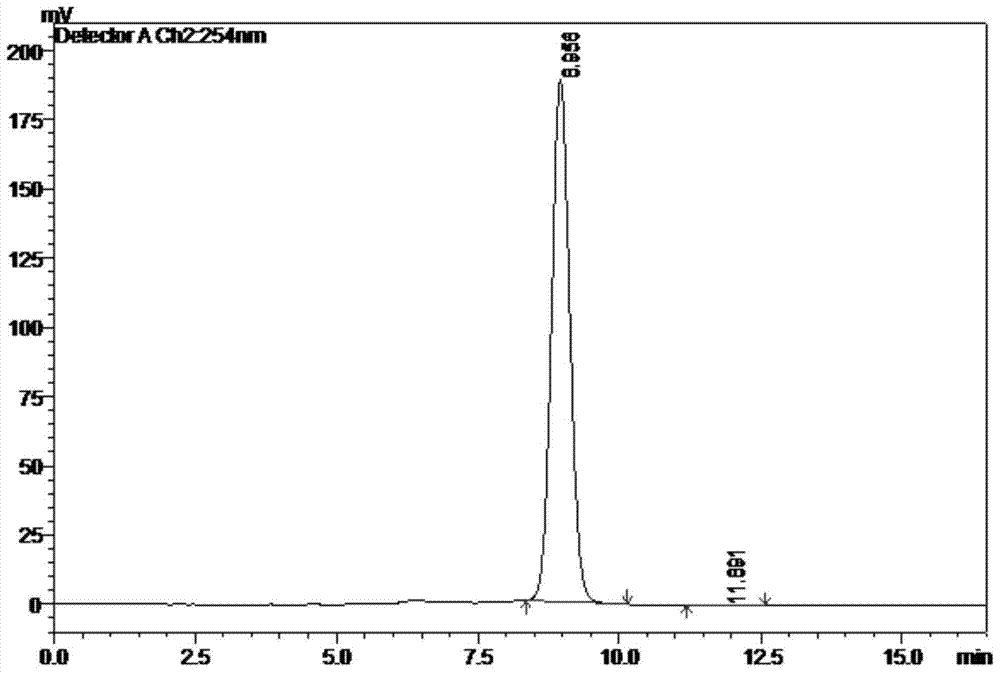
[0052] The component of peak 4 in the chromatogram is collected from Example 1, and after the solvent is removed by rotary evaporation, the mobile phase is dissolved again, shaken up as the sample to be tested, injected into the high performance liquid chromatograph, and the mobile phase is eluted, and then According to the peak time of the chromatogram, record the chromatogram, see attached figure 2 , the peak data in the figure are shown in Table 2: Peak No. 1 is 2,6-...
Embodiment 3
[0055] Example 3: 2,6-diaminotriptycene enantiomer (9R,10R)-9,10-dihydro-9,10-[1,2]benzanthracene-2,6-diamine (Peak 5 components in the separation chromatography of embodiment 1) detection
[0056] Instrument and chromatographic conditions:
[0057] High-performance liquid chromatography, the chromatographic column is Chiralpak IC, 0.46cm I.D. × 15cm L, 1.0 μ L; the mobile phase is ethanol: diethylamine = 100: 0.1 (V / V); the column temperature is 35 ° C; the flow rate is 1.0mL / min; detection wavelength is 254nm.
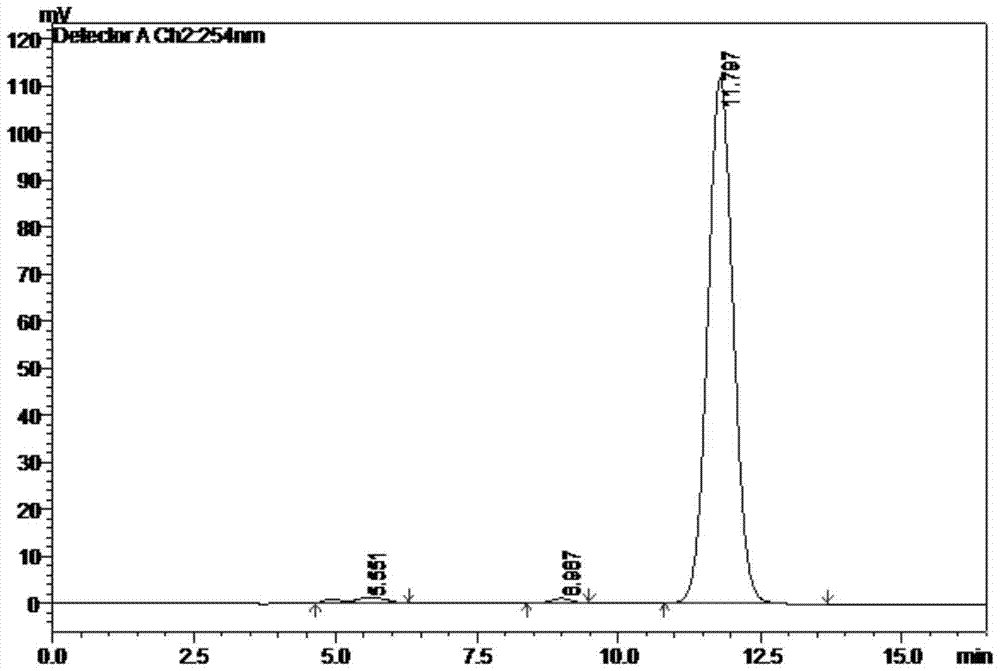
[0058] The component of peak 5 in the chromatogram is collected from Example 1, after the solvent is removed by rotary evaporation, the mobile phase is dissolved again, shaken up as the sample to be tested, injected into the high performance liquid chromatograph, and the mobile phase is eluted, according to Chromatogram peak time, record chromatogram, see attached image 3 , the peak data in the figure are shown in Table 3: the No. 2 main peak is 2,6-diaminotripty...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com