Applications of ROCK2 inhibitor in preparing medicines for treating inflammatory bowel diseases
An inflammatory bowel disease and inhibitor technology, which is applied to ROCK2 inhibitors and the application field of preparing inflammatory bowel disease medicines, can solve the problems of unclear immune response effect and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
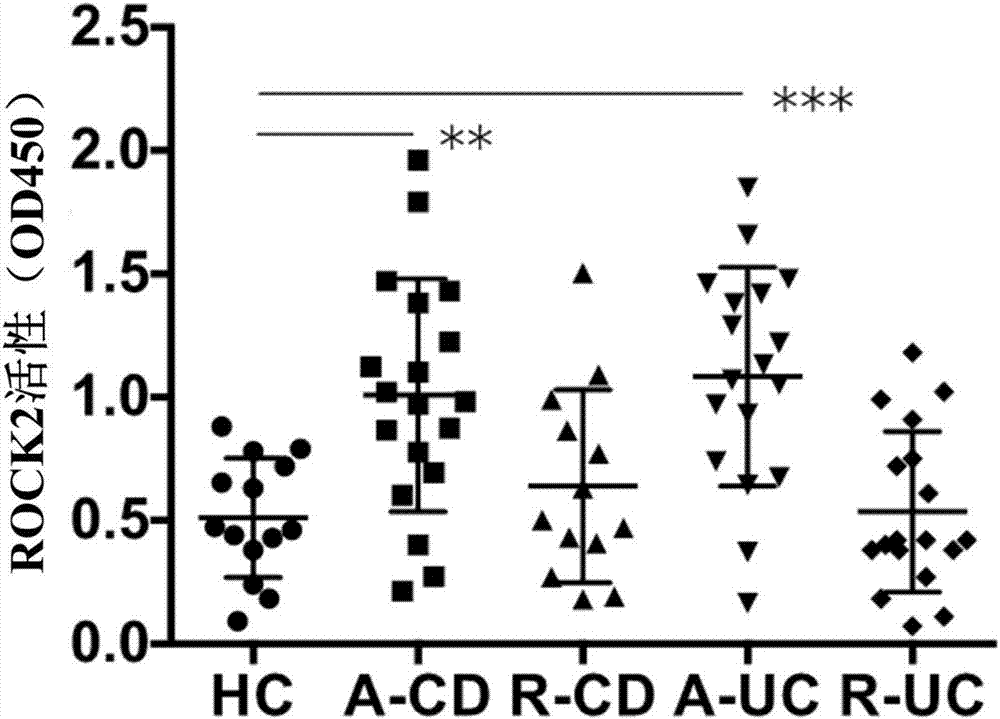
[0016] Example 1. Determination of ROCK2 activity
[0017] 1.1 Extraction of total tissue protein
[0018] 1) Put the intestinal mucosal tissue into an EP tube, add an appropriate amount of IP lysate into the tube, and grind it on ice with a grinding rod until no formed tissue can be seen, then place it on ice for 2 hours.
[0019] 2) After fully lysing, centrifuge, centrifugation conditions: 12000rpm, 4°C, 5min. After the centrifugation is over, carefully take out the EP tube, and carefully transfer the supernatant liquid to another EP tube with a pipette gun.
[0020] 1.2 Determination of protein concentration
[0021] 1) Make a standard curve according to the instructions of the BCA kit. The protein standard curve formulation table is as follows:
[0022] Hole number 0 1 2 3 4 5 6 7 Protein standard solution (μl) 0 1 2 4 8 12 16 20 Deionized water (μl) 20 19 18 16 12 8 4 0 Corresponding protein content (μg) 0 0.5 1.0 ...
Embodiment 2
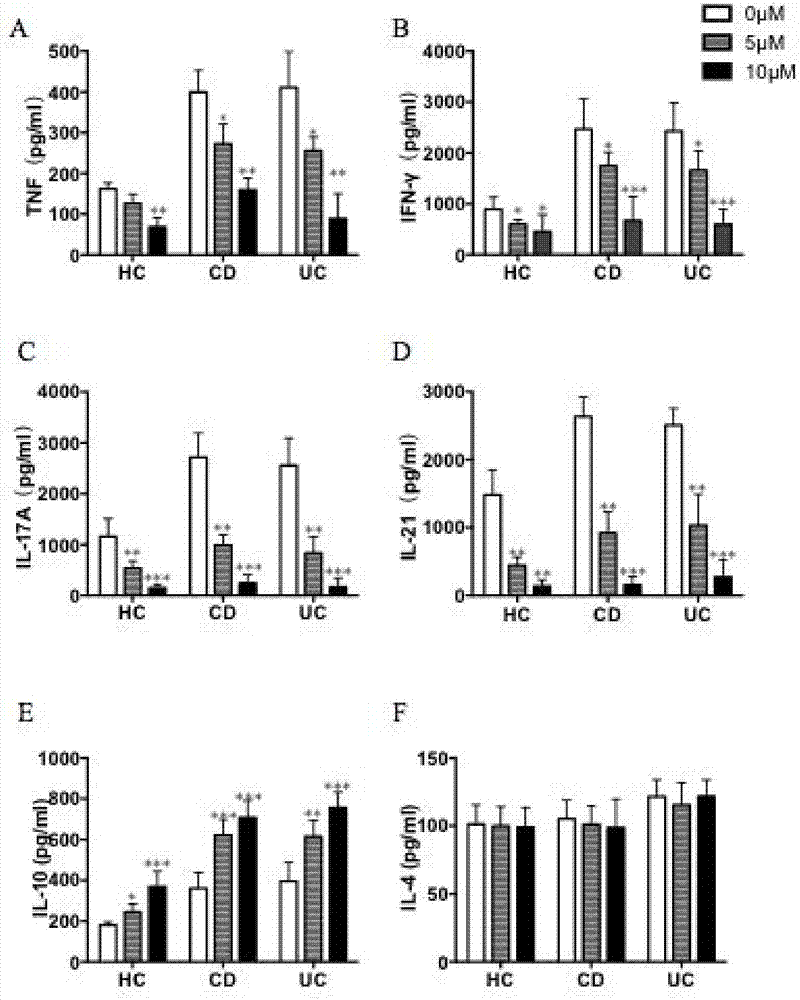
[0036] Example 2. Enzyme-linked immunosorbent assay (enzyme-linkedimmuno sorbentassay, ELISA)
[0037] (1) Dilute 200× CaptureAntibody (coating antibody) to 1× in the sample tank with coating buffer. Use a pipette to transfer 50 μl of 1× CaptureAntibody to a 96-well plate, and shake the 96-well plate slowly to ensure that the liquid surface covers the bottom of the well. Place in 4°C refrigerator overnight.
[0038] (2) On the second day, the liquid in the wells was removed, and the wells were washed 3 times with buffer solution, each time the buffer solution stayed in the wells for 3 minutes.
[0039] (3) Transfer 100 μl of blocking solution (blocking buffer) to a 96-well plate with a pipette, and shake in a shaker at room temperature for 1 hour.
[0040] (4) After washing the wells according to step (2), dilute the protein standard with blocking solution according to the following concentration gradient: 1000ng, 500ng, 250ng, 125ng, 64ng, 32ng and 16ng. Transfer 50 μl of ...
Embodiment 3
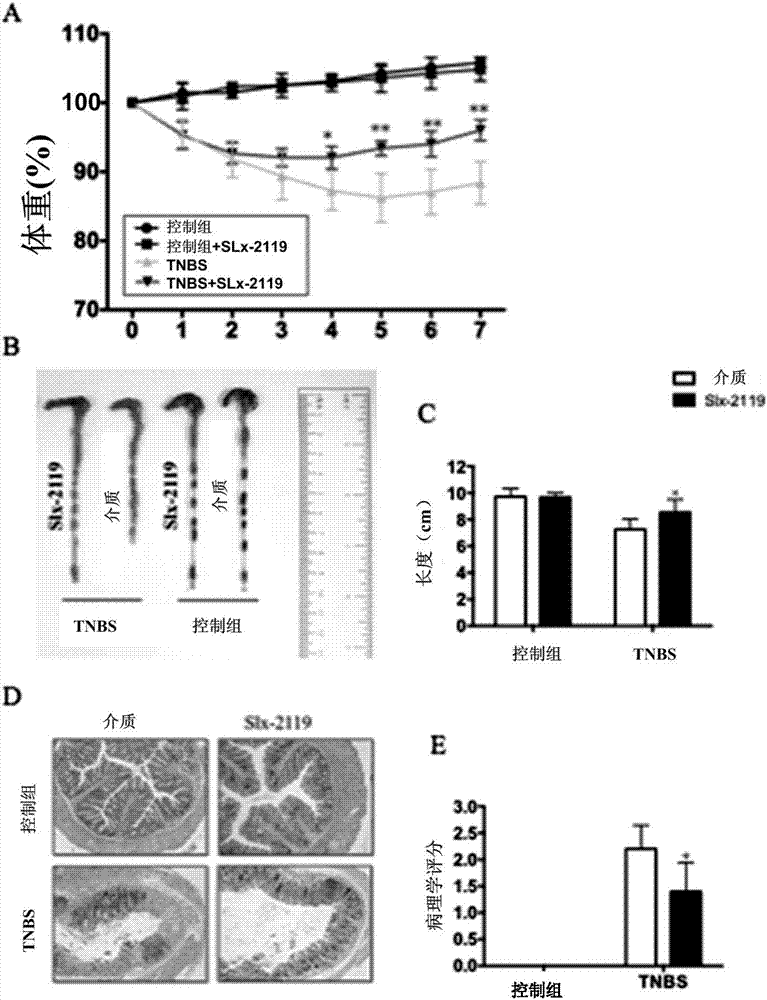
[0046] Example 3. TNBS-induced colitis model in mice and in vivo treatment with SLx-2119
[0047] (1) The Balb / c mice to be tested were fasted for 24 hours and divided into four groups: A, B, C, and D groups; wherein, the Balb / c mice were a commercially available commodity.
[0048] (2) Mix 5% TNBS and 100% ethanol at a ratio of 1:1 to form an appropriate amount of TNBS mixture, and store it in a refrigerator at 4°C in the dark.
[0049] (3) Prepare 1.25% sodium pentobarbital and anesthetize the mice to be tested.
[0050] (2) Grab the tail of the anesthetized mouse, slowly insert the enema tube connected with the syringe from the anus of the mouse to 4 cm, and slowly inject 150 μl of TNBS mixture into the mice of the two groups A and B, while the other group Two groups were infused with 150μl 50% ethanol.
[0051] (4) Lift the mouse upside down for 30 seconds, put the mouse back into the cage, and restore the normal food and water supply.
[0052] (5) After waking up, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com