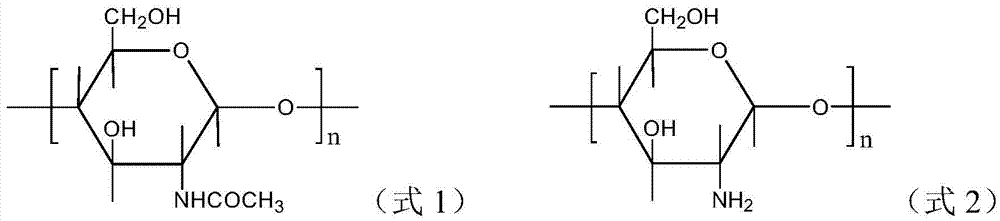
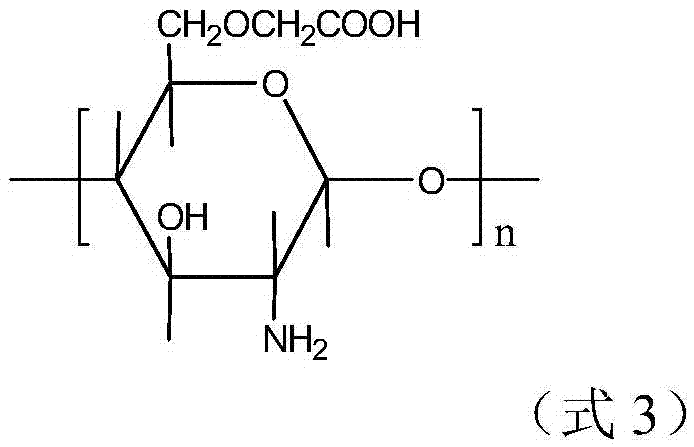
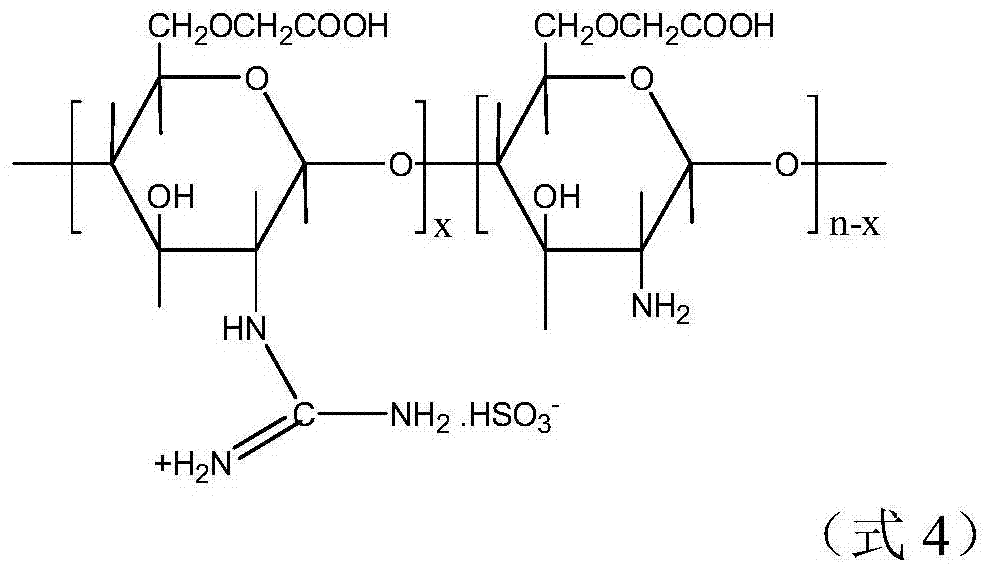
An amphoteric carboxymethyl chitosan guanidine salt derivative and a preparing method thereof
A technology of carboxymethyl chitosan and its derivatives, which is applied in the field of amphoteric carboxymethyl chitosan guanidinium salt derivatives and its preparation, can solve problems affecting application, long reaction time, complicated process, etc., and achieve improved water solubility , The preparation method is simple and the price is cheap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of amphoteric carboxymethyl chitosan guanidinium salt derivatives
[0044] 100 mg of carboxymethyl chitosan was added to 100 ml of deionized water, and mechanically stirred for 30 min under a water bath condition of 25° C. to obtain a carboxymethyl chitosan aqueous solution with a concentration of 1 mg / ml;
[0045] According to the molar ratio of thiourea trioxide and carboxymethyl chitosan is the ratio of 10:1, under the condition of 25 ℃, thiourea trioxide is slowly added in the described carboxymethyl chitosan aqueous solution in 30min, Keep the reaction temperature in the reactor at 25°C, and react for 60 minutes to obtain the second mixed solution;
[0046] Filter the second mixed solution and put it into a dialysis bag, tie both ends of the dialysis bag tightly and put it into deionized water for dialysis treatment, change the water every four hours, and put the dialysate into the dialysis bag after changing the water eight times. Freeze in a refriger...
Embodiment 2 to 8
[0049] Except the contents shown in Table 1, amphoteric carboxymethyl chitosan guanidinium salt derivatives were prepared in the same manner as in Example 1.
[0050] Raw material consumption and preparation process parameters in the preparation of amphoteric carboxymethyl chitosan guanidinium salt derivative in the embodiment 1-8 of table 1
[0051]
[0052] Sample elemental analysis result and guanidino substitution degree in table 2 raw material, embodiment 1-8
[0053]
Embodiment 9
[0055] Application of Amphoteric Carboxymethyl Chitosan Guanidine Derivatives in Antibacterial Properties
[0056] The test is carried out according to the plate coating method in the 2002 edition of the "Disinfection Technical Specifications" issued by the Ministry of Health. The culture medium is mixed evenly according to the ratio of 1:9, and then poured into the petri dish while it is hot. The culture dish needs about 20ml of culture medium solution. cultured in an incubator for 36 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com