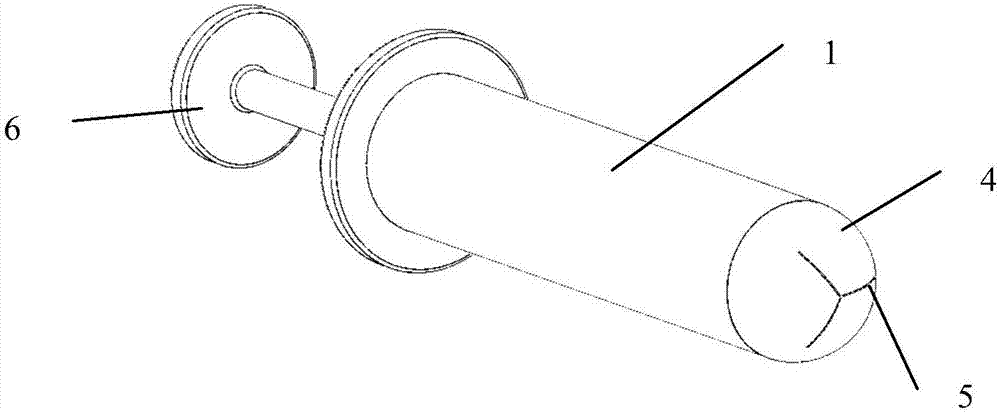
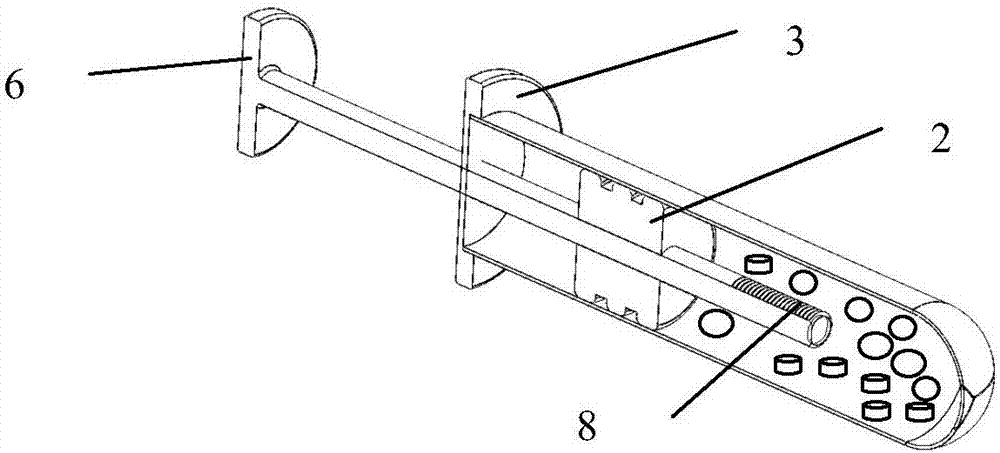
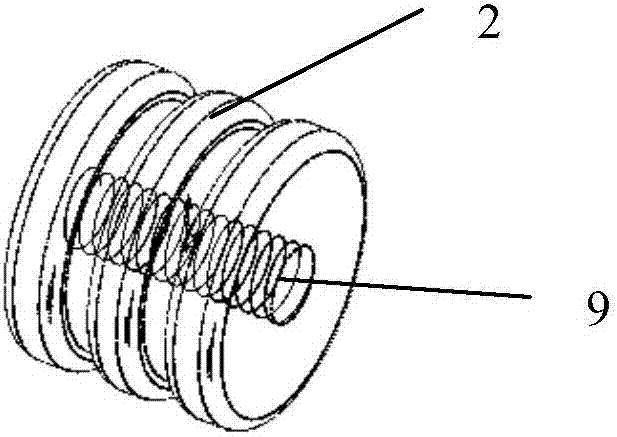
First-aid hemostatic device
A hemostatic material and drug placement technology, applied in the field of medical devices, can solve the problems of decreased hemostatic effect, organ damage, pain, etc., and achieve the effects of wide application site, strong hemostatic performance, and improved effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: The hemostatic material is a polyvinyl alcohol sponge. The average pore diameter of the custom-made sponge is about 0.02 mm, the original size is about 10 mm in diameter, and about 30 mm in height.
Embodiment 2
[0037] Example 2: The hemostatic material is polyvinyl alcohol sponge. The average pore size of the custom-made sponge is about 0.02 mm. The original size is about 10 mm in diameter and about 30 mm in height. The compressed cylindrical size is about 11 mm in diameter and about 7 mm in height.
Embodiment 3
[0038] Embodiment 3: The hemostatic material is a polyvinyl alcohol sponge, and the custom-made sponge has an average pore diameter of about 0.02mm, an original size diameter of about 10mm, and a height of about 30mm. The sponge is soaked in chitosan solution (1%) for 2min, compressed and dried at room temperature, compressed Rear cylindrical size: diameter 11mm, height 7mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com