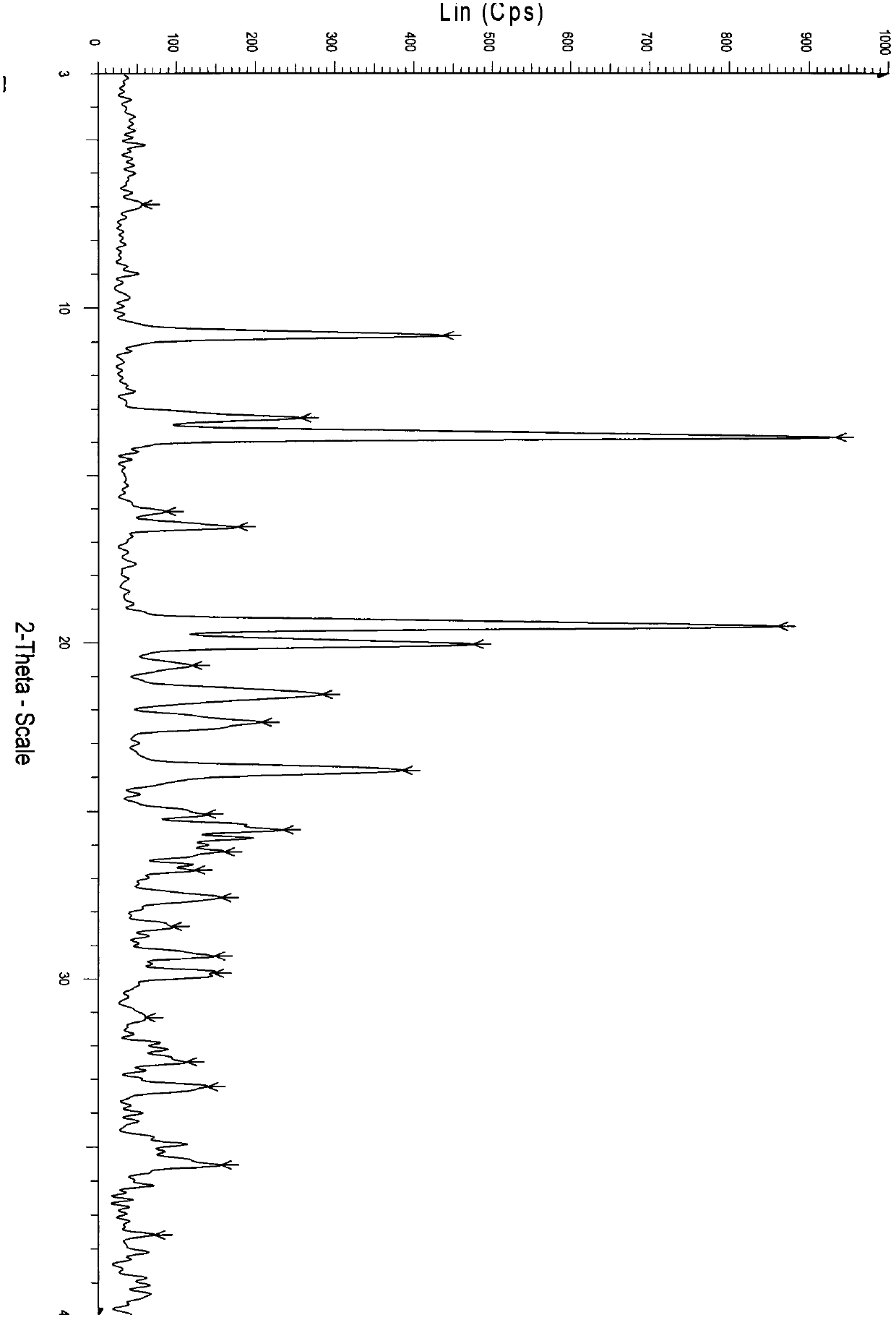
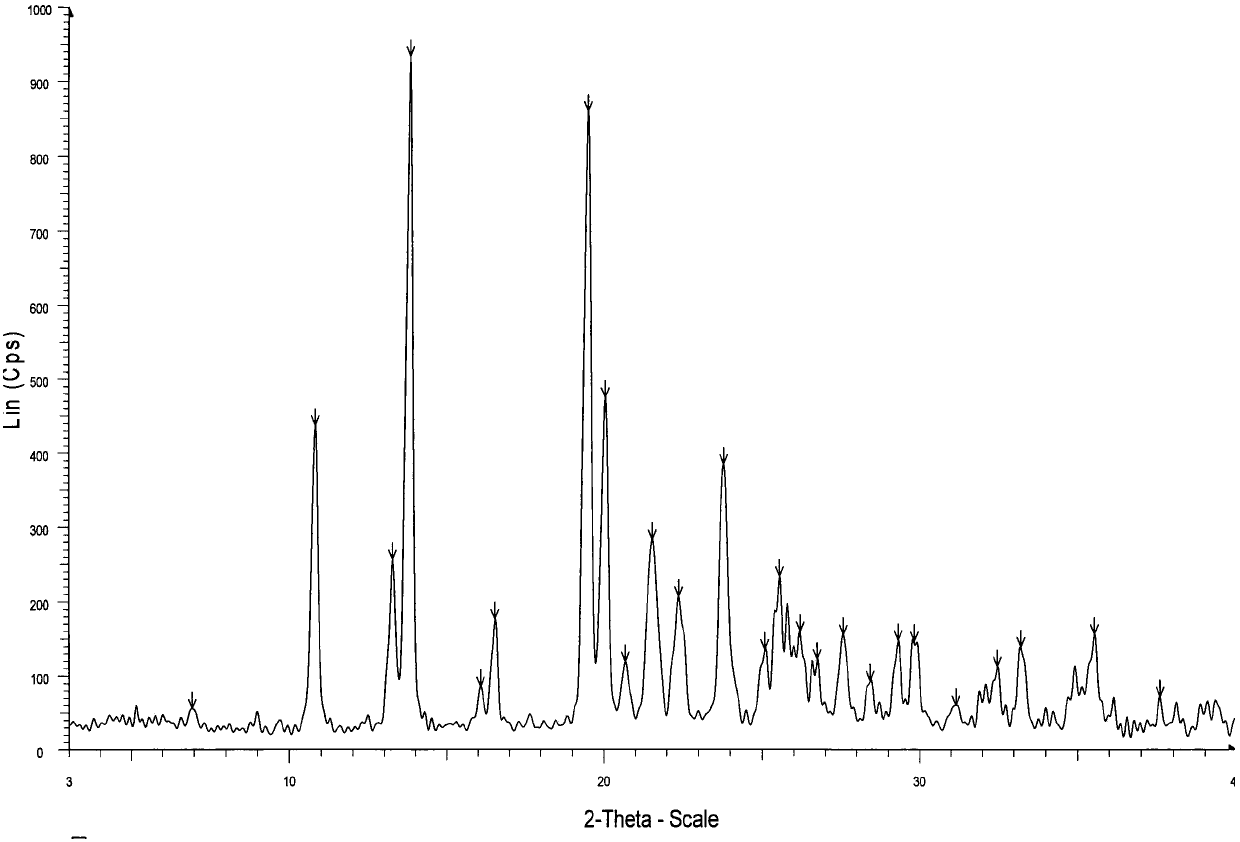
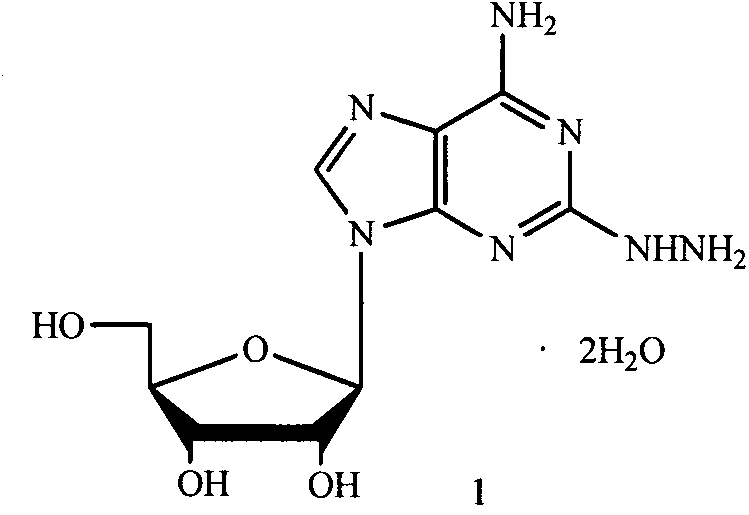
2-hydrazino adenosine and preparation method thereof
A technology of hydrazinoadenosine and hydrazinoadenosine dihydrate, which is applied in the field of medicine and can solve the problems of cumbersome preparation and purification, high cost, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] In the method for preparing 2-hydrazinoadenosine dihydrate 1 of the present invention, the amount of hydrazine hydrate is 0.5 to 10 times, preferably 1 to 2 times, the volume of the compound of formula II.
[0027] In the preparation method of the present invention, the reaction solvent may be one or more mixtures of water, methanol, ethanol, n-propanol or isopropanol. Among them, water, methanol or ethanol may be used as a preferable reaction solvent, and methanol is particularly preferable. The amount of the reaction solvent used is 1-10 times, preferably 1-5 times, particularly preferably 2-3 times the volume of the compound of formula II.
[0028] In the preparation method of the present invention, the reaction temperature is 0-100°C, preferably 50-90°C, particularly preferably 80-90°C.
[0029] In the preparation method of the present invention, the reaction time is 1-10 hours, preferably 2-8 hours, particularly preferably 5-7 hours.
[0030] A crystalline form o...
Embodiment 1
[0037] Example 1: Synthesis of 2-hydrazinoadenosine dihydrate
[0038]
[0039] Add 50g of 2',3',5'-triacetyl-2-chloroadenosine (IIa) into 100mL of hydrazine hydrate, and react at a temperature of 80-90°C for 5h. Cool down to 10-20°C and stir for 1h. Filter, wash with water and ethanol, suck dry, and dry under reduced pressure at 50°C for 6 hours to obtain 31.9 g of off-white solid. Yield 81.9%. Moisture 10.9%, purity (HPLC) 97.4%.
Embodiment 2
[0040] Example 2: Synthesis of 2-hydrazinoadenosine dihydrate
[0041] Add 50g of 2',3',5'-triacetyl-2-chloroadenosine (IIa) into 50mL of hydrazine hydrate and 100mL of ethanol, and react at a temperature of 80-90°C for 5h. Cool down to 10-20°C and stir for 1h. Filter, wash with ethanol, suck dry, and dry under reduced pressure at 50°C for 6 hours to obtain 36.8 g of off-white solid. Yield 94.5%. Moisture 10.8%, purity (HPLC) 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com