Method for recycling and preparing superfine nickel powder from nickel-hydrogen cell
A nickel-metal hydride battery and metal nickel powder technology, applied in the field of hydrometallurgy and powder metallurgy, can solve the problems of high energy consumption and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
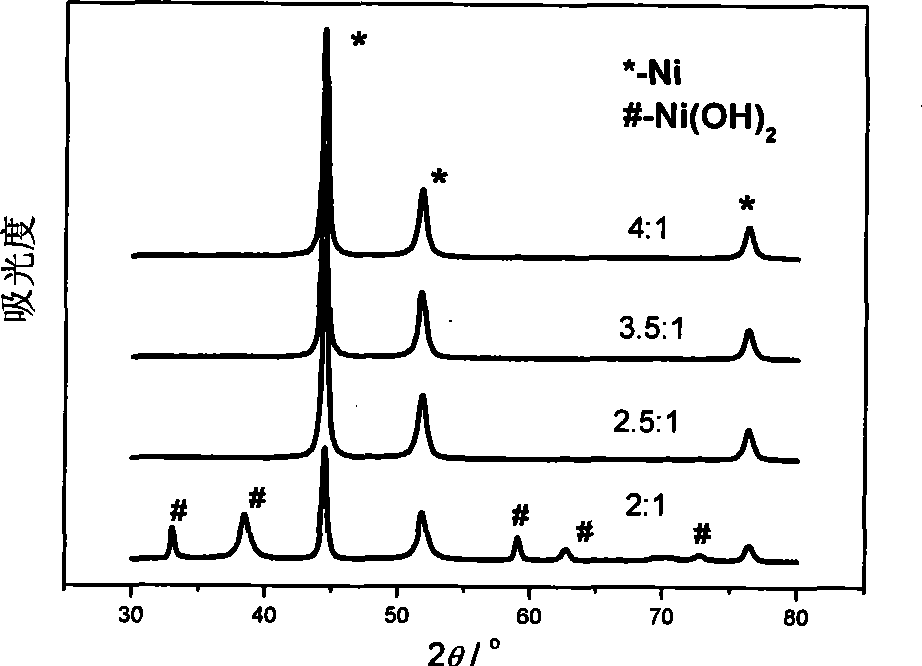
Image
Examples
Embodiment 1
[0035] Leaching stage: Prepare 3.2mol / L sulfuric acid solution, measure 150ml and pour it into a 500ml beaker, put the beaker into a constant temperature water bath, and adjust the temperature to 85°C; weigh 40g of powdery nickel metal hydride battery positive electrode waste, pour it into In the sulfuric acid solution, keep stirring with a magnetic stirrer, and after reacting for 60 minutes, filter to remove the filter residue, and make the filtrate to a constant volume of 250ml. The specific content of each element in the leachate is measured by diacetyl oxime spectrophotometry and flame atomic absorption spectrometer as follows:
[0036] element Ni co Na Mg CD Cu Fe Ca Zn mn Leaching solution (g / L) 77.1 6.02 1.00 2.08 3.86 1.04 0.83 0.40 0.87 0.19
[0037] The leaching rate of nickel is calculated to be about 75.1%.
Embodiment 2
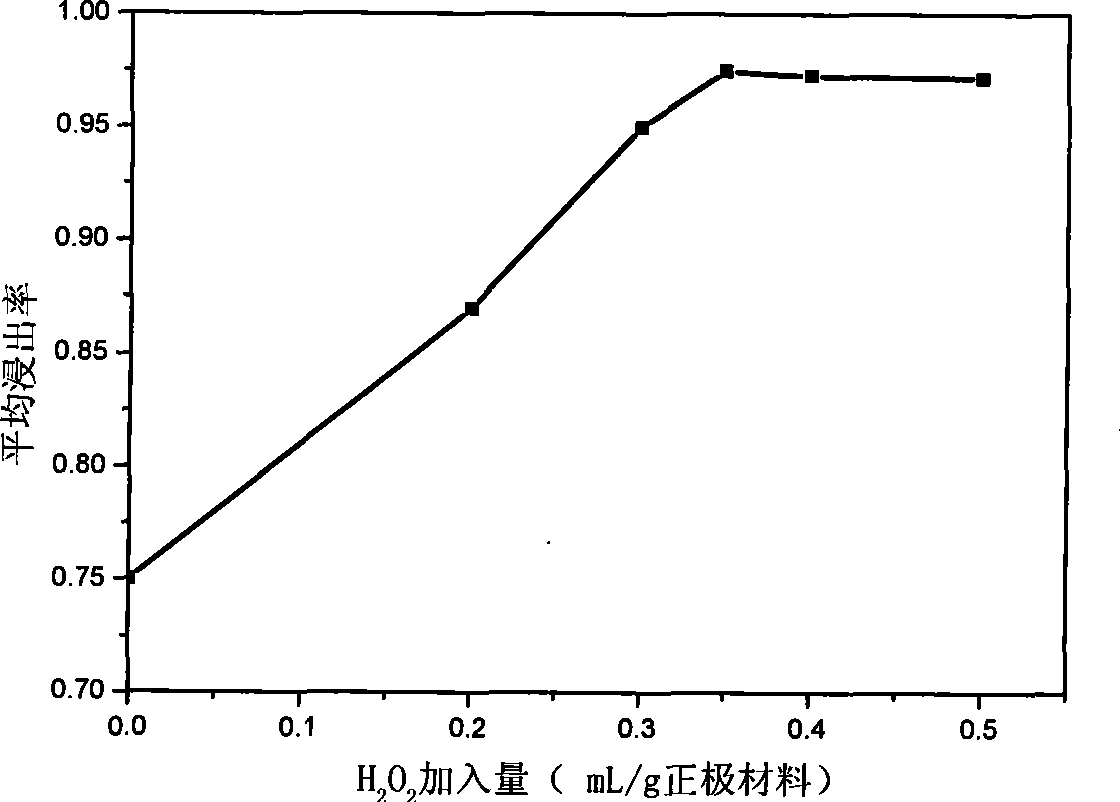
[0039] Leaching stage: prepare 3.2mol / L sulfuric acid solution, measure 150ml and pour it into a 500ml beaker, measure 11.7ml hydrogen peroxide solution (containing H 2 o 2 30%) into the same beaker, put the beaker into a constant temperature water bath, and adjust the temperature to 85°C; weigh 40g of powdered Ni-MH battery positive electrode waste, pour it into the mixed solution, keep stirring with a magnetic stirrer, and react for 60 minutes , filtered to remove the filter residue, and the filtrate was constant volume 250ml. The specific content of each element in the leachate is measured by diacetyl oxime spectrophotometry and flame atomic absorption spectrometer as follows:
[0040] element Ni co Mg Na CD Cu Fe Ca Zn mn Leaching solution (g / L) 102.1 6.62 2.18 1.01 5.02 1.22 0.93 0.48 0.91 0.21
[0041] The leaching rate of nickel is calculated to be about 99.6%.
Embodiment 3
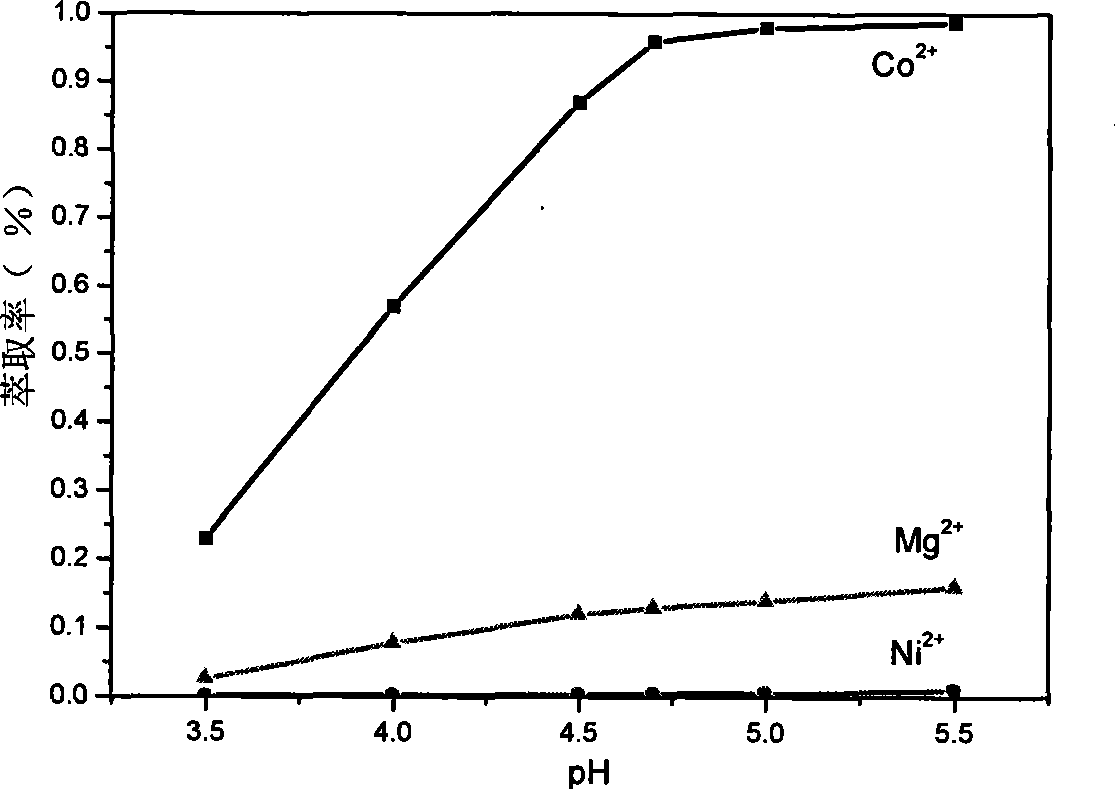
[0043] P204 impurity removal stage: adjust the pH value of the sulfuric acid leaching solution to about 3.0 with NaOH, and then use P204 to extract and remove impurities after oxidation. , the organic phase was first saponified with NaOH, the saponification rate was 75%, and the secondary countercurrent extraction was carried out, and the extraction equilibrium pH value was 3.5. After the extraction, the aqueous phase solution, the specific content of each element measured by dimethylglyoxime spectrophotometry, flame atomic absorption spectrometer and plasma emission spectrometry (ICP) is as follows:
[0044] element Ni co Na Mg CD Cu Fe Ca Zn mn After P204 decontamination
[0045] It can be seen from the above table that Cd, Cu, Fe, Ca, Zn, Mn, etc. basically all enter the organic phase, but some nickel and cobalt also enter the organic phase, and the loss of nickel is about 4.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com