Fc-region variants with modified fcrn-binding and methods of use
A variant and antibody technology, applied in the field of Fc region variants with altered FCRN binding and its use, can solve problems such as increasing half-life and reducing clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1241] expression and purification
[1242] Transient transfection in the HEK293-F system
[1243] Using the HEK293-F system (Invitrogen), according to the manufacturer's instructions, by transient transfection with the corresponding plasmids (eg, encoding the heavy chain and modified heavy chain and the corresponding light chain and modified light chain), monospecific and bispecific specific antibody. In short, serum-free FreeStyle TM HEK293-F cells (Invitrogen) cultured in suspension in 293 expression medium (Invitrogen), with the corresponding expression plasmid and 293fectin TM Or fectin (Invitrogen) mixture transfection. For 2L shake flasks (Corning), HEK293-F cells were mixed with 1*10 6 The density of cells / mL was inoculated in 600mL and heated at 120rpm, 8% CO 2 Incubation. The next day, the cells were incubated with approximately 1.5*10 6 A cell density of 2 cells / mL was transfected with approximately 42 mL of the following mixture: A) 20 mL of Opti-MEM (Invi...
Embodiment 2
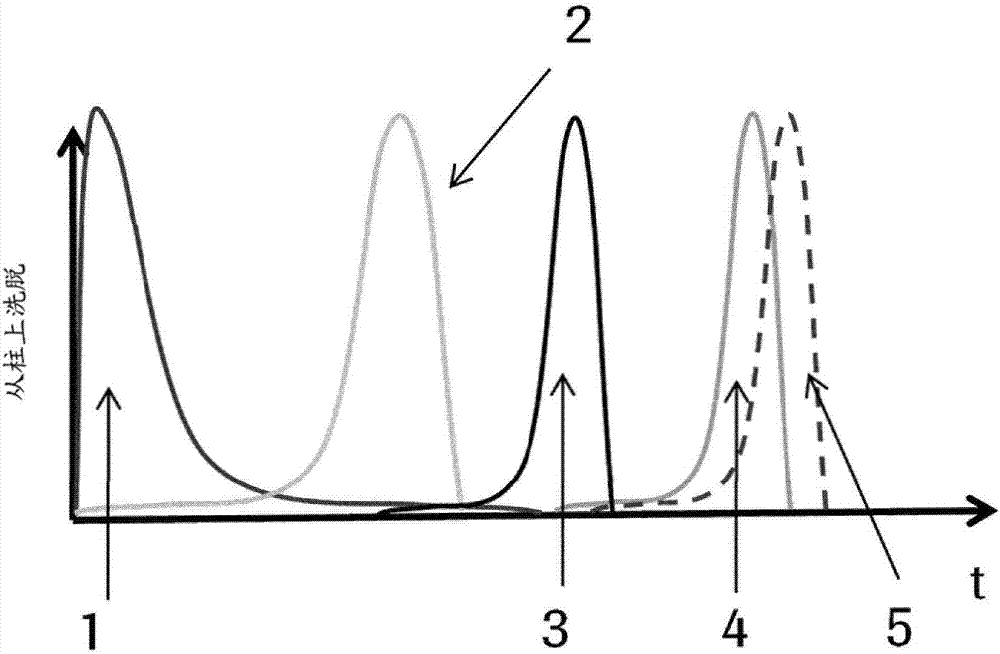
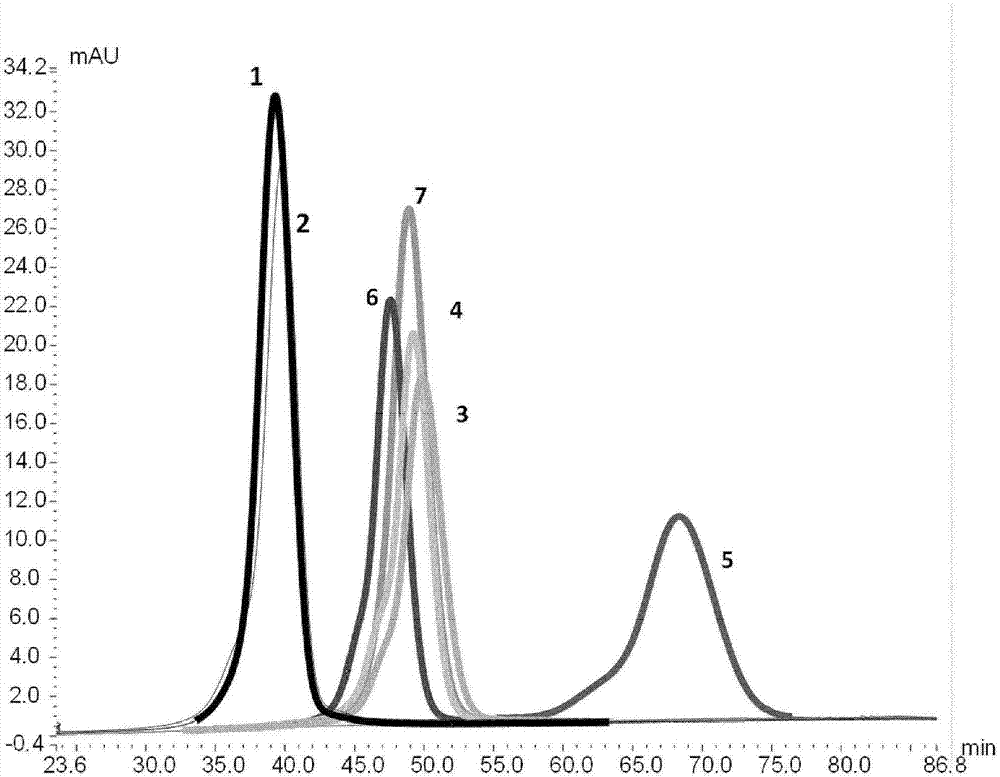
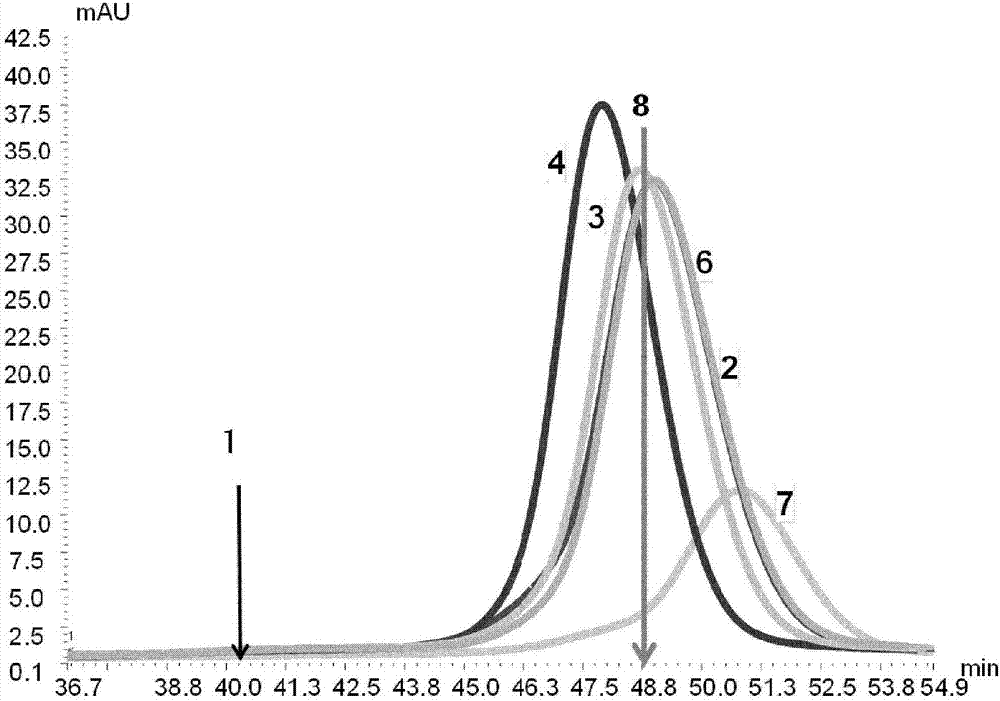
[1250] FcRn Chromatography
[1251] Coupling to Streptavidin Sepharose:
[1252] 1 gram of Streptavidin Sepharose (GE Healthcare) was added to the biotinylated and dialyzed receptors and incubated for 2 hours with shaking. Receptor derivatized Sepharose was packed in a 1 mL XK column (GE Healthcare).
[1253] Chromatography using FcRn affinity columns:
[1254] condition:
[1255] Column size: 50mm x 5mm
[1256] Bed height: 5cm
[1257] Loading capacity: 50μg sample
[1258] Equilibration buffer: 20mM MES with 150mM NaCl, adjusted to pH 5.5
[1259] Elution buffer: 20mM Tris / HCl with 150mM NaCl adjusted to pH 8.8
[1260] Elution: 7.5CV equilibration buffer, 100% elution buffer in 30CV, 10CV elution buffer.
[1261]
[1262]
[1263]
[1264]
[1265]
[1266]
[1267]
[1268]
[1269]
[1270]
[1271]
[1272]
[1273]
[1274]
[1275]
[1276]
[1277]
[1278]
[1279]
[1280]
[1281]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com